Would You Like To Join Ask Sawal
Ask Sawal is a fast growing question and answer discussion forum.
25 lakh+ questions were answered by our Ask Sawal Members.
Each day 1000s of questions asked& 1000s of questions answered.
Ask any question and get answer from 5 Lakh+ Ask Sawal Members.
Constant moderation and reporting option makes questions and answers spam free.
And also, we have free blogging platform. Write an article on any topic.
We have 10000+ visitors each day. So a beneficial platform for link building.
We are allowing link sharing. Create backlinks to your blog site or any site.
Gain extra passive income by sharing your affiliate links in articles and answers.
Health Hazards Of Sodium Hydroxide
- Contact with extremely high concentrations of sodium hydroxide can result in severe burns to the eyes, skin, digestive system, or lungs, which can lead to permanent damage or death.
- Mucous membranes in the nose, throat, lungs, and bronchial system may be damaged. Even tiny doses can cause significant harm.
- Burns the skin and damages the eyes. The respiratory tract is irritated. Irritation of the mucous membranes in the nose.Avoid coming into touch with your eyes, skin, or clothes. Gases, fumes, dust, mist, vapour, and aerosols should not be inhaled. Wear safety glasses, gloves, and clothes. See Section 8 for further information. Acids should not be mixed. Do not eat, drink, smoke, or use personal products when handling chemical substances.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
You May Like: Molecular Shape Of Ccl4
As A Nucleophilic Reagent
The hydroxide ion is intermediate in nucleophilicity between the fluoride ion F, and the amide ion NH2. The hydrolysis of an ester
- R1COR2 + H2O R1COH + HOR2
also known as saponification is an example of a nucleophilic acyl substitution with the hydroxide ion acting as a nucleophile. In this case the leaving group is an alkoxide ion, which immediately removes a proton from a water molecule to form an alcohol. In the manufacture of soap, sodium chloride is added to salt out the sodium salt of the carboxylic acid this is an example of the application of the common ion effect.
Other cases where hydroxide can act as a nucleophilic reagent are amide hydrolysis, the Cannizzaro reaction, nucleophilic aliphatic substitution, nucleophilic aromatic substitution, and in elimination reactions. The reaction medium for KOH and NaOH is usually water but with a phase-transfer catalyst the hydroxide anion can be shuttled into an organic solvent as well, for example in the generation of the reactive intermediate dichlorocarbene.
Sodium Hydroxide In Other Industrial Manufacturing Uses
Sodium hydroxide is used in many other industrial and manufacturing processes. It is used to manufacture rayon, spandex, explosives, epoxy resins, paints, glass and ceramics. It is also used in the textile industry to make dyes, process cotton fabric and in laundering and bleaching, as well as in metal cleaning and processing, oxide coating, electroplating and electrolytic extracting.
Recommended Reading: Holt Geometry Answer Key
What Is Sodium Hydroxide
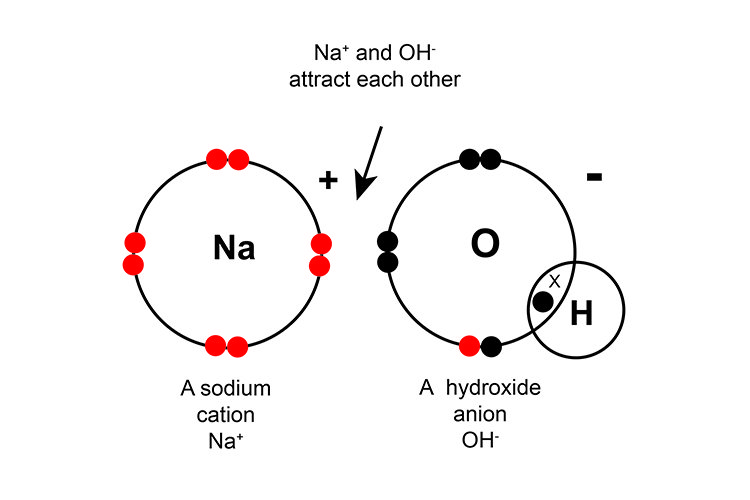
Sodium Hydroxide is a solid ionic compound. It is also known as caustic soda, Iye, sodium hydrate or soda lye.
It is a co-product of chlorine production. In its pure form, it is crystalline solid, colourless in nature. This compound is highly water-soluble and consists of sodium cations and hydroxide anions.NaOH absorbs moisture from the air. It is highly corrosive and can cause severe skin burn and irritation to eyes and other body parts.
It generates a high level of heat and so is always created by mixing the compound into the water, not the vice versa. In cosmetics, this inorganic compound is used as a buffering agent. It can also control the PH levels. The PH of sodium hydroxide is 13.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Also Check: Fsa Warm Ups Grade 5
Amount Of Naoh To Make Sodium Hydroxide Solution
Prepare solutions of sodium hydroxide using this handy reference table which lists the amount of solute that is used to make 1 L of base solution. Follow these lab safety guidelines:
- Don’t touch sodium hydroxide! It is caustic and could cause chemical burns. If you do get NaOH on your skin, immediately rinse it with a large volume of water. Another option is to neutralize any base on the skin with a weak acid, such as vinegar, and then rinse with water.
- Stir the sodium hydroxide, a little at a time, into a large volume of water and then dilute the solution to make one liter. Add sodium hydroxide to waterdo not add water to solid sodium hydroxide.
- Be sure to use borosilicate glass and consider immersing the container in a bucket of ice to keep the heat down. Inspect the glassware prior to use to make sure it is free from any cracks, scratches or chips that would indicate a weakness in the glass. If you use a different type of glass or weak glass, there’s a chance the temperature change could cause it to shatter.
- Wear safety goggles and gloves since there is a chance the sodium hydroxide solution could splash up or the glassware could break. Concentrated solution of sodium hydroxide are corrosive and should be handled with care.
Reactions With Dissolved Metals
When sodium hydroxide reacts with certain dissolved metals, it forms a solid. This reaction is commonly used to remove dissolved metals from a solution, particularly if they are toxic.
When a reaction involves transforming soluble ions into an insoluble solid it is known as a precipitation reaction. Sodium hydroxide can be used to facilitate this reaction in many transition metals, like copper sulphate .
Transition metals are very soluble in water and form coloured solutions when dissolved. Copper sulphate, for example, turns the solution a characteristic light blue. Zinc, on the other hand, produces a white colour.
If sodium hydroxide is introduced into an aqueous solution containing a soluble transition metal, the transition metal is displaced from its compound. This process is called a displacement reaction, and it happens because sodium is a much more reactive metal.
Once the transition metal has been displaced, an insoluble transition metal hydroxide is formed. In the case of copper sulphate, sodium hydroxide displaces the copper and copper hydroxide 2) is formed. Instead of dissolving, this compound precipitates and appears as a light blue solid in the liquid. At this point, the solution turns colourless and solid copper hydroxide sits at the bottom.
Read Also: Algebra Connections Chapter 3 Answers
What Are The Uses Of Sodium Hydroxide
As a popular strong base, sodium hydroxide has a wide range of uses across many different industries. It is commonly used as a pH regulator because it can neutralise acids.
It also reacts with weak acids, like hydrogen sulphide, on a given substrate to produce sodium salts, which can be easily removed. This process is called caustic washing, and it is used to remove sulphurous impurities from things like crude oil. But NaOH is much more than a pH regulator.
A Generic Biodiesel Flowsheet
A generic flowsheet for a continuous plant is shown in Figure 1.
Figure 1. Generic biodiesel production flowsheet.
Solid sodium hydroxide is mixed with > 99.9% methanol. Sodium hydroxide solutions cannot be used, as the water in the solutions is undesirable, as mentioned above. This is also the reason for the relatively high purity of the methanol .
Downstream of the reactor, the settler at its most simple design is an unmixed vessel that gives the reaction mixture sufficient time to separate by gravity, as glycerol = 1.26) is substantially denser than biodiesel . It separates the reaction mixture into a biodiesel-rich phase and a glycerol-rich phase .
Washing is usually achieved using water, resulting in wastewater of typically very high BOD and COD . The process is often a two-stage wash with freshwater at each stage. It reduces a range of contaminants in the BRP, including soap, methanol, and catalyst.
Polishing refers to the last step in the process in which various low-level contaminants are removed. In particular, metal ions from the catalyst and various soaps are removed. It is usually achieved using an ion exchange resin, notably Rohm and Haass BD10Dry, specifically developed for this application.
DR.James G. Speight, in, 2017
Recommended Reading: Linear Algebra Span Definition
In Cement Mixes Mortars Concrete Grouts
Sodium hydroxide is used in some cement mix plasticisers. This helps homogenise cement mixes, preventing segregation of sands and cement, decreases the amount of water required in a mix and increases workability of the cement product, be it mortar, render or concrete.
See: Sodium hydroxide test for flavonoids
Summer-winter heat storage
EMPA researchers are experimenting with concentrated sodium hydroxide as the thermal storage or seasonal reservoir medium for domestic space-heating. If water is added to solid or concentrated sodium hydroxide , heat is released. The dilution is exothermic chemical energy is released in the form of heat. Conversely, by applying heat energy into a dilute sodium hydroxide solution the water will evaporate so that the solution becomes more concentrated and thus stores the supplied heat as latent chemical energy.
Neutron Moderator
Seaborg Technologies is working on a reactor design in which NaOH is used as a neutron moderator.
Sodium Hydroxide In Cleaning & Disinfectant Products
Sodium hydroxide is used to manufacture soaps and a variety of detergents used in homes and commercial applications. Chlorine bleach is produced by combining chlorine and sodium hydroxide. Drain cleaners that contain sodium hydroxide convert fats and grease that can clog pipes into soap, which dissolves in water.
Don’t Miss: Holt Geometry Textbook Answers
Uses Of Sodium Hydroxide
- It is used in the manufacturing of detergents and soaps
- It is used in the production of bleach-like chlorine
- It is used in drain cleaners
- It is used in the removal of heavy metals from the water by the municipal water treatment facility
- It is used in food preservatives to prevent bacteria and mould growth
- It is used for canning
- It is used in papermaking and paper recycling process.
Sodium Hydroxide In Energy
In the energy sector, sodium hydroxide is used in fuel cell production. Fuel cells work like batteries to cleanly and efficiently produce electricity for a range of applications, including transportation materials handling and stationary, portable and emergency backup power applications. Epoxy resins, manufactured with sodium hydroxide, are used in wind turbines.
Don’t Miss: Geometry Dash Hack Steam
Common Uses And Potential Hazards
Sodium hydroxide has a great variety of household and industrial uses. It is the active ingredient in drain cleaners such as Drano® because it breaks up and dissolves the greasy mass that is responsible for drain blockages. It is also an ingredient in many other household products, including oven cleaners, metal polishes, and hair straighteners. Sodium hydroxide is also used in the preparation of homemade and processed foods. It is used in the preparation of soft drinks, chocolate, ice creams, caramel coloring, and cocoa. Hominy, a starchy food similar to grits, is made by soaking corn kernels in a solution of sodium hydroxide in water. Bakers glaze pretzels and German lye rolls with a weak lye solution before baking them. The lye gives baked goods a crisp crust. Some people use lye to cure olives.
Sodium Hydroxide Chemical Formula
This compound is ionic in nature and consists of Na+ or OH- ions. Na is a cation and the hydroxide is an anion. To prepare the compound, you should perform the electrolysis process where aqueous NaCl gives sodium hydroxide and gases, flakes, pellets etc.
2 NaCl + 2 H2O 2 NaOH + Cl2 + H2
Sodium Hydroxide Properties
This is an odourless chemical compound and highly soluble in polar solvent like ethanol, water, methanol etc. This is not soluble in organic solvents. When NaOH is dissolved in water, it will undergo exothermic reaction and results in aqueous solution that is transparent, free from odor and used in laboratories as a common base.
As a stronger base, it readily combines with acids to form HCl and corresponding salts. Due to its hygroscopic features, it could absorb the CO2 and water from the air. The compound is widely used in paper industries, textile industries, pH regulation or industrial cleaning etc. it can be used for food industries too for a variety of purposes. The compound is quite corrosive in nature and decomposes quickly. Tt may severe irritation to eyes and permanent blindness in the worst cases.
Don’t Miss: What Is An Example Of Movement In Geography
What Personal Protective Equipment Is Needed When Working With Sodium Hydroxide
Eye/Face Protection: Wear chemical safety goggles. A face shield may also be necessary.
Skin Protection: Wear chemical protective clothing e.g. gloves, aprons, boots. Suitable materials include: butyl rubber, natural rubber, neoprene rubber, nitrile rubber, polyvinyl chloride, Vitonî, Vitonî/butyl rubber, Barrierî – PE/PA/PE, Silver Shieldî – PE/EVAL/PE, Trellchemî HPS, Trellchemî VPS, Saranexîââ¢, Tychemî BR/LV, Tychemî Responderî CSM, Tychemî TK. Recommendations are NOT valid for very thin natural rubber, neoprene rubber, nitrile rubber, and PVC gloves .
Respiratory Protection:
Up to 10 mg/m3: Any supplied-air respirator operated in a continuous-flow mode* OR Any powered air-purifying respirator with a high-efficiency particulate filter*. Any air-purifying, full-facepiece respirator with an N100, R100, or P100 filter OR Any self-contained breathing apparatus with a full facepiece OR Any supplied-air respirator with a full facepiece.
*Causes eye irritation or damage eye protection needed.
APF = Assigned Protection Factor
Recommendations apply only to National Institute for Occupational Safety and Health approved respirators. Refer to the NIOSH Pocket Guide to Chemical Hazards for more information.
CLOSE ALL
Add a badge to your website or intranet so your workers can quickly find answers to their health and safety questions.
Sodium Hydroxide In Wood & Paper Products
In many paper making processes, wood is treated with a solution containing sodium sulfide and sodium hydroxide. This helps dissolve most of the unwanted material in the wood, leaving relatively pure cellulose, which forms the basis of paper. In the paper recycling process, sodium hydroxide is used to separate the ink from the paper fibers allowing the paper fibers to be reused again.
Sodium hydroxide is also used to refine raw materials for wood products such as cabinets and furniture and in wood bleaching and cleaning.
Also Check: Renate Blauel Children
What Else Does Sodium Hydroxide React With
Reactions involving sodium hydroxide do not stop here. Some other examples of what sodium hydroxide reacts with include, but are not limited to:
- Irreversible saponification reaction with fats, which is used in the manufacture of soap, shampoos and other cosmetic products
- Interaction with multi-atomic alcohols to form alcoholates, white crystalline substances that dissolve well in water
- Disproportionation reaction with non-metals, such as sulphur or chlorine, which involves a single substance getting oxidised and reduced
ReAgent supplies sodium hydroxide in 1kg and 25kg bags as well as a range of concentrations. All of our products are backed by a 100% quality guarantee, so you can buy NaOH with confidence. Order online today or contact our friendly team for any further information.
Disclaimer
The blog on chemicals.co.uk and everything published on it is provided as an information resource only. The blog, its authors and affiliates accept no responsibility for any accident, injury or damage caused in part or directly from following the information provided on this website. We do not recommend using any chemical without first consulting the Material Safety Data Sheet which can be obtained from the manufacturer and following the safety advice and precautions on the product label. If you are in any doubt about health and safety issues please consult the Health & Safety Executive .
Related Posts
What Is The Abbreviation For Sodium Hydroxide Naoh
Sodium Hydroxide can be abbreviated as NaOH. What is the meaning of NaOH abbreviation? The meaning of NaOH abbreviation is Sodium Hydroxide. What is NaOH abbreviation? One of the definitions of NaOH is Sodium Hydroxide. What does NaOH mean? NaOH as abbreviation means Sodium Hydroxide. What is shorthand of Sodium Hydroxide?
Also Check: What Effect Did Geography Have On The Way Greece Developed
Basic Salts Containing Hydroxide
In some cases the products of partial hydrolysis of metal ion, described above, can be found in crystalline compounds. A striking example is found with zirconium. Because of the high oxidation state, salts of Zr4+ are extensively hydrolyzed in water even at low pH. The compound originally formulated as ZrOCl2·8H2O was found to be the chloride salt of a tetrameric cation 8+ in which there is a square of Zr4+ ions with two hydroxide groups bridging between Zr atoms on each side of the square and with four water molecules attached to each Zr atom.
The mineral malachite is a typical example of a basic carbonate. The formula, Cu2CO32 shows that it is halfway between copper carbonate and copper hydroxide. Indeed, in the past the formula was written as CuCO3·Cu2. The crystal structure is made up of copper, carbonate and hydroxide ions. The mineral atacamite is an example of a basic chloride. It has the formula, Cu2Cl3. In this case the composition is nearer to that of the hydroxide than that of the chloride CuCl2·3Cu2. Copper forms hydroxyphosphate , arsenate , sulfate , and nitrate compounds. White lead is a basic lead carbonate, 2·Pb2, which has been used as a white pigment because of its opaque quality, though its use is now restricted because it can be a source for lead poisoning.