What Are The Uses Of Ferric Oxide
Ferric oxide is used in paints and coatings, inks, plastics, rubber products and glass as a pigment and/or UV blocking agent. It is also used as a polishing agent for glass, diamonds and precious metals. This compound also finds use as a component in magnets, as a dental abrasive, and as a process regulator or catalyst in the manufacture of other products.
Valence States Of Iron And Oxygen In Feo2hx
Fig. 1
Representative XAS and XRS spectra of iron oxide compounds. a PFY-XAS spectra at Fe K-edge of iron oxide compounds at room temperature. Black, olive, blue, and cyan lines: Fe0, Fe2+O, Fe3+2O3, and CaFe4+O3 at ambient conditions, respectively light and dark magenta lines: Py-FeO2 at 53 and 81GPa, respectively red line: Py-FeO2Hx at 133GPa gray arrow: the link from the dashed outline to the inset. Inset: the area zoomed for the dashed outline in Fig. . b XRS spectra of Py-FeO2Hx at 110GPa. Circles: experimental data shaded areas: fitted peaks
Production Of Iron Oxide:
Iron oxide is a product obtained from the oxidation of iron. In laboratories, it is prepared by electrolyzing sodium bicarbonate solution, an inert electrolyte, along with an iron anode.
The deriving hydrated iron oxide, which is written here as FeOH, dehydrates at around 200 °C. The reaction is as follows:
Read Also: Geometry Eoc Fsa Practice Test (calculator Portion)
What Are The Properties And Uses Of Iron
It is a smooth, greyish metal, rusting in the humid weather. Iron is an enigma it quickly rusts but it remains the most significant of all metals. Of all the metal processed today, 90 per cent is iron. The bulk is used for steel construction, used in construction engineering and manufacturing.
|
Related Elements |
Mechanisms Of Iron Regulation

Human iron homeostasis is regulated at two different levels. Systemic iron levels are balanced by the controlled absorption of dietary iron by enterocytes, the cells that line the interior of the intestines, and the uncontrolled loss of iron from epithelial sloughing, sweat, injuries and blood loss. In addition, systemic iron is continuously recycled. Cellular iron levels are controlled differently by different cell types due to the expression of particular iron regulatory and transport proteins.
Recommended Reading: Example Of Span Linear Algebra
Oxygen And Chalcogen Compounds
Several oxides of cobalt are known. Green cobalt oxide has rocksalt structure. It is readily oxidized with water and oxygen to brown cobalt hydroxide 3). At temperatures of 600700 °C, CoO oxidizes to the blue cobalt oxide , which has a spinel structure. Black cobalt oxide is also known. Cobalt oxides are antiferromagnetic at low temperature: CoO and Co3O4 , which is analogous to magnetite , with a mixture of +2 and +3 oxidation states.
What Can I Do With My Eit Certification
Engineer-in-training certification is an intermediate and necessary step to licensure. You cannot stamp or seal engineering documents, as a PE could, with an EIT certification. EIT certification doesnt grant you any special powers it simply indicates youve passed the FE exam and that you are on your way to become a licensed professional engineer . An EIT certification also works as a proof to employers that you possess the technical competence required to pass a comprehensive engineering exam.
Read Also: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Study Area And Seawater Sampling
Seawater was sampled during the ANT-XXXI/3 expedition on-board the R.V. Polarstern in March 2016 at 25 m depth at three different stations: BIO_1, BIO_2 and BIO_3. BIO_1 and BIO_3 were located across the Drake Passage whereas BIO_2 was situated close to the Western Antarctic Peninsula . At each site, seawater was pumped through a trace-metal cleaned polyethylene tubing using a
Also Check: What Does Abiotic Mean In Biology
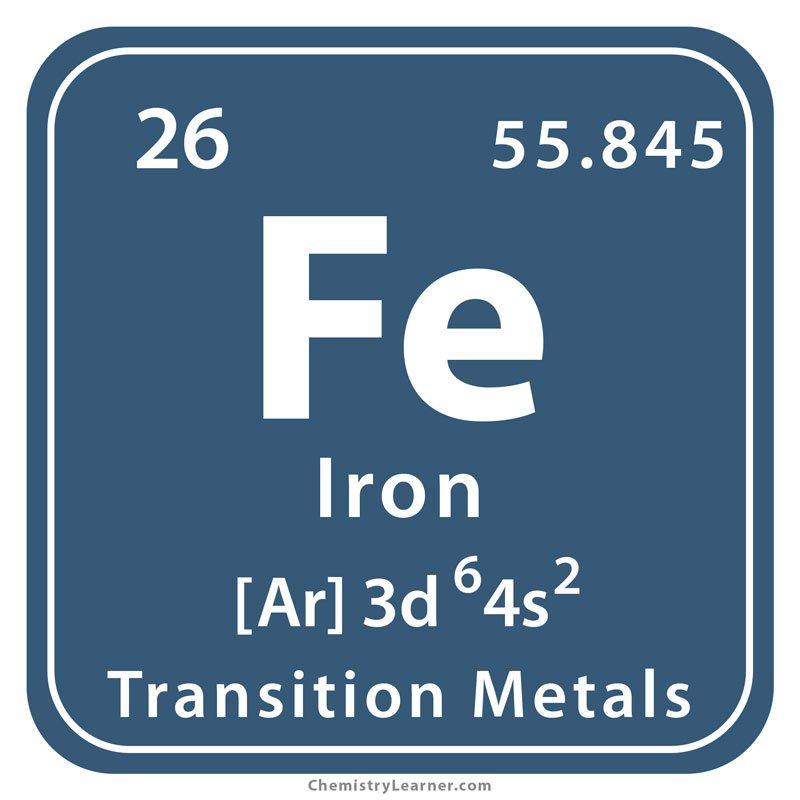
What Does Fe Stand For
What does FE mean? This page is about the various possible meanings of the acronym, abbreviation, shorthand or slang term: FE.
Filter by:
What does FE mean?
- iron, Fe, atomic number 26
- a heavy ductile magnetic metallic element is silver-white in pure form but readily rusts used in construction and tools and armament plays a role in the transport of oxygen by the blood
Popularity rank for the FE initials by frequency of use:
Couldn’t find the full form or full meaning of FE?
Maybe you were looking for one of these abbreviations:
Discuss these FE abbreviations with the community:
Report Comment
We’re doing our best to make sure our content is useful, accurate and safe.If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we’ll take care of it shortly.
Theoretical Study Predicts Ironcarbon Quadruple Bond
Computational research by a team in India predicts that a quadruple bond could form between iron and carbon in the compound C3.1
Multiple bonding between main group elements has been demonstrated up to a maximum of three bonds. However, researchers have proposed quadruple bonds in C2 and the isoelectronic equivalents CN+, BN and CB-.
A theoretical and experimental study by scientists in China in 2019 reported the formation of a quadruple bond between boron and iron in the B3- anion.2 That proved the unprecedented characterisation of the FeB quadruple bond and motivated us to investigate the possibility of the formation of a quadruple bond between a transition metal and carbon, says Ankur Guha, of Cotton University.
As carbon atoms are isoelectronic with boron anions Guhas team decided to investigate if a quadruple bond could form between carbon and iron in the molecule C3. Our high level calculations reveal the unprecedented formation of a true FeC quadruple bond with very high bond strength, says Guha. The findings lend weight to the theory of single electron transmutation, which predicts that after gaining an electron, an atom will act like its neighbour with the same number of electrons.
Guhas team hopes to expand on their study by collaborating with an experimental research group to confirm the bond.
Also Check: Why Do I Hate My Birthday Psychology
Sample Synthesis And Characterization
The five samples of the pyrite-type phase have been synthesized and further probed by X-ray spectroscopic techniques in this study . Iron superoxide samples of FeO2 or FeO2Hx were synthesized from goethite or 57Fe-enriched hematite mixture with O2 and water H2O, respectively, at 18002200K under target pressures in laser-heated DACs at 16IDB, 13ID-D and High Pressure Synergetic Consortium of the Advanced Photon Source of Argonne National Laboratory. In order to avoid any signal contamination from other phases in the sample and to assure complete transition, the whole starting materials of goethite or hematite have been laser-heated repeatedly for 23h at target pressures to synthesize a pure pyrite-structured FeO2Hx phase.
What Is The Fundamentals Of Engineering Exam
The NCEES Fundamentals of Engineering exam is the first of the two exams engineers must pass in order to become licensed professional engineers in the United States.
Engineering college seniors or working engineers must pass the FE exam and then gain four years of engineering work experience before becoming eligible to take the PE exam. Upon passing the PE exam, the examinee becomes a licensed professional engineer . A licensed professional engineer is someone who has the legal right to stamp and seal engineering documents that go out to the public.
The NCEES FE exam is a ~5.5-hour, 110-question long, multiple-choice test that covers the majority of the courses seen in an engineering undergraduate curriculum. The exam is taken on a computer at a Pearson Vue test center location. You are only allowed to bring an approved calculator, and you are provided a PDF of equations and tables that you will refer to during the exam. No cheat sheets or notes are allowed.
Recommended Reading: Massachusetts Bay Colony Geography And Climate
Will I Get Paid More If I Pass The Fe
Remember passing the FE exam is simply a required, intermediate step before becoming eligible to take-pass the PE exam. Will passing the FE help you get a raise? It depends.
If you are a civil engineer, it is expected for you to pass the FE exam from the very beginning of your career. It’s likely your employer will hold off on giving you a promotion solely based on the fact that you are not an EIT yet. So for civils, passing the FE will make you more money in the sense that you will not be held back from getting a promotion because you hadn’t passed the FE exam. What about the PE exam, will that make me more money? Yes, it is standard practice for civil engineers to get a raise immediately after passing the PE exam. The standard raise immediately after passing the PE exam is an additional $2,000-$5,000 per year in compensation. Additionally, you will now be eligible for project engineer or team manager positions after passing the PE exam which tend to pay $10K+ more than entry-level EIT positions.
Statue Of Liberty: Changing Colors
The Statue of Liberty is a landmark every American recognizes. The Statue of Liberty is easily identified by its height, stance, and unique blue-green color . When this statue was first delivered from France, its appearance was not green. It was brown, the color of its copper skin. So how did the Statue of Liberty change colors? The change in appearance was a direct result of corrosion. The copper that is the primary component of the statue slowly underwent oxidation from the air. The oxidation-reduction reactions of copper metal in the environment occur in several steps. Copper metal is oxidized to copper oxide , which is red, and then to copper oxide, which is black
Coal, which was often high in sulfur, was burned extensively in the early part of the last century. As a result, sulfur trioxide, carbon dioxide, and water all reacted with the CuO
These three compounds are responsible for the characteristic blue-green patina seen today. Fortunately, formation of the patina created a protective layer on the surface, preventing further corrosion of the copper skin. The formation of the protective layer is a form of passivation, which is discussed further in a later chapter.
Figure 1.
Perhaps the most familiar example of corrosion is the formation of rust on iron. Iron will rust when it is exposed to oxygen and water. The main steps in the rusting of iron appear to involve the following . Once exposed to the atmosphere, iron rapidly oxidizes.
Figure 2.Figure 3.
Also Check: How To Find Ksp Chemistry
Effective Atomic Number In Coordination Compounds
In the above definition, we explain effective atomic number concept. This concept explains the stability and the possibility of complex compound formation. According to this concept, only that complex compound can be formed that will attain the noble gas configuration.
D-block element atomic number
Zinc – 30
Krypton – 36
The Nobel gas close to this series is 36. All these elements are less stable than the krypton. Therefore, all these above-mentioned elements will try to attain this electronic configuration. For this noble gas configuration, these elements will form a complex compound with different types of the ligand.
What Is The Fe Exam And Why You Should Take It
In this article, we take a high level look of what is the NCEES Fundamentals of Engineering Exam, sometimes referred to as the EIT exam. We go over what are the steps to become a licensed professional engineer in the United States and how each step to licensure works. Additionally, we touch on why you should take the FE exam and what it means for your career to become an engineer-in-training and later on a licensed professional engineer .
You May Like: Eoc Algebra 1 Practice Test With Answers 2015
Magmatic Magnetite Ore Deposits
Occasionally graniteand ultrapotassicigneous rocks segregate magnetite crystals and form masses of magnetite suitable for economic concentration. A few iron ore deposits, notably in Chile, are formed from volcanic flows containing significant accumulations of magnetite phenocrysts. Chilean magnetite iron ore deposits within the Atacama Desert have also formed alluvial accumulations of magnetite in streams leading from these volcanic formations.
Some magnetite skarn and hydrothermal deposits have been worked in the past as high-grade iron ore deposits requiring little beneficiation. There are several granite-associated deposits of this nature in Malaysia and Indonesia.
Other sources of magnetite iron ore include metamorphic accumulations of massive magnetite ore such as at Savage River, Tasmania, formed by shearing of ophioliteultramafics.
Another, minor, source of iron ores are magmatic accumulations in layered intrusions which contain a typically titanium-bearing magnetite often with vanadium. These ores form a niche market, with specialty smelters used to recover the iron, titanium and vanadium. These ores are beneficiated essentially similar to banded iron formation ores, but usually are more easily upgraded via crushing and screening. The typical titanomagnetite concentrate grades 57% Fe, 12% Ti and 0.5% V2O
Chemistry End Of Chapter Exercises
Mg or Ca
Fe or Zn
Ag or Pt
You May Like: What Does Fault Mean In Geography
Melting And Boiling Points
The melting and boiling points of iron, along with its enthalpy of atomization, are lower than those of the earlier 3d elements from scandium to chromium, showing the lessened contribution of the 3d electrons to metallic bonding as they are attracted more and more into the inert core by the nucleus however, they are higher than the values for the previous element manganese because that element has a half-filled 3d sub-shell and consequently its d-electrons are not easily delocalized. This same trend appears for ruthenium but not osmium.
The melting point of iron is experimentally well defined for pressures less than 50 GPa. For greater pressures, published data still varies by tens of gigapascals and over a thousand kelvin.
Synchrotron Mssbauer Spectroscopy Experiments

The SMS spectra of 57Fe-enriched Py-phase and Fe2O3 samples were collected at beamline 16-IDD, APS, ANL. A monochromatic X-ray beam with an energy of 14.41keV, a bandwidth of 2meV, and 57µm in FWHM was used to excite the 57Fe nuclei in the sample. An avalanche photodiode detector was used to collect the time-delayed SMS signals in the forward direction with a typical collection time of ~24h for each pressure. Pressure before and after each measurement was determined based on the Raman spectra of the diamond anvils where the sample contacted. Py-phase samples were synthesized from the assemblages of Fe2O3 and H2O or O2 approximately at 90100GPa and 2000K. After the SMS spectrum of the sample was collected, a thin stainless-steel foil or a platelet of sulfate heptahydrate was placed on the downstream side of the DAC to serve as a reference for the isomer shift measurements. Mössbauer hyperfine parameters, including quadrupole splitting, isomer shift, and magnetic hyperfine field of the samples were derived using the CONUSS program.
Also Check: My Hrw Algebra 2
Foundations Of Modern Chemistry
In 1774, Antoine Lavoisier used the reaction of water steam with metallic iron inside an incandescent iron tube to produce hydrogen in his experiments leading to the demonstration of the conservation of mass, which was instrumental in changing chemistry from a qualitative science to a quantitative one.
Iron Oxide Structure Fe2o3
The above image describes the structure of the Iron oxide. Fe2O3 is the chemical formula of Iron oxide which has three oxygen atoms, two iron atoms. The oxidation state of Fe2O3 is +3. The bond formation between oxygen and iron depends on the difference in electronegativity between these two atoms. Iron is metal whereas oxygen is non-metal. Therefore, such bonds are called an Ionic bond.
Atoms:
| O |
Read Also: Algebra 1 Age Word Problems