With Varying Ph Temperature And Salinity: Caco3 Scaling In Swimming Pools
In contrast to the open equilibrium scenario above, many swimming pools are managed by addition of sodium bicarbonate to about 2 mM as a buffer, then control of pH through use of HCl, NaHSO4, Na2CO3, NaOH or chlorine formulations that are acidic or basic. In this situation, dissolved inorganic carbon is far from equilibrium with atmospheric CO2. Progress towards equilibrium through outgassing of CO2 is slowed by
In this situation, the dissociation constants for the much faster reactions
- H2CO3 H+ + HCO3 2 H+ + CO23
allow the prediction of concentrations of each dissolved inorganic carbon species in solution, from the added concentration of HCO3 . Addition of HCO3 will increase CO23 concentration at any pH. Rearranging the equations given above, we can see that =
- HCO3]
The solubility product for CaCO3 and the dissociation constants for the dissolved inorganic carbon species are all substantially affected by temperature and salinity, with the overall effect that max increases from freshwater to saltwater, and decreases with rising temperature, pH, or added bicarbonate level, as illustrated in the accompanying graphs.
Chemical And Physical Characteristics Of Calcium Its Interaction With Water
Why is it stored in a sealed container?
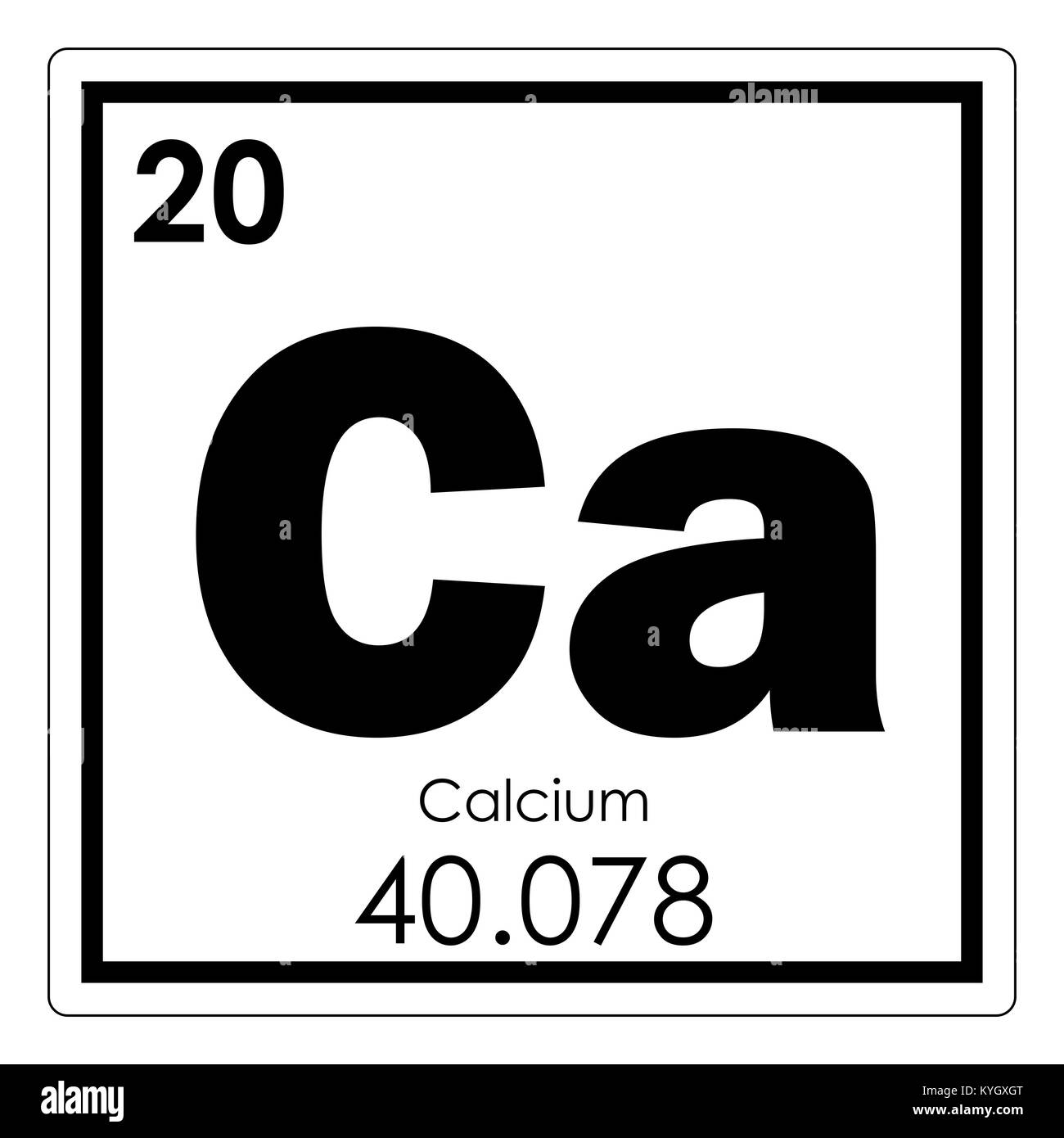
Calcium is found in the fourth large period, second group, main sub-group, with the atomic number 20. The atomic mass of calcium, according to the periodic table, is 40.08. The formula of the highest oxide is CaO. The symbol of the element is Ca, after the first two letters of the word calcium.
Naturally Occurring Nuclides That Are Not Primordial
Some unstable isotopes which occur naturally are not primordial, as they must be constantly regenerated. This occurs by , or by such processes as geonuclear transmutation . Other examples of common naturally occurring but non-primordial nuclides are isotopes of , , and , which are all daughters of uranium decay and are found in uranium ores. The stable isotope 40Ar is actually more common as a radiogenic nuclide than as a primordial nuclide, forming almost 1% of the earth’s , which is regenerated by the of the extremely long-lived radioactive primordial isotope , whose half-life is on the order of a billion years and thus has been generating argon since early in the Earth’s existence.
A similar radiogenic series is derived from the long-lived radioactive primordial nuclide . These nuclides are described as geogenic, meaning that they are decay or fission products of uranium or other actinides in subsurface rocks. All such nuclides have shorter half-lives than their parent radioactive primordial nuclides. Some other geogenic nuclides do not occur in the of 232Th, 235U, or 238U but can still fleetingly occur naturally as products of the of one of these three long-lived nuclides, such as , which makes up about 1014 of all natural .
Don’t Miss: How To Find My Biological Father
Interesting & Fun Facts About Calcium
How Can Calcium Hydroxide Be Prepared

Calcium hydroxide is formed by the action of water on calcium oxide, also called slaked lime, Ca2. A small proportion of it dissolves when combined with water, forming a solution known as limewater, the remainder remaining in a suspension called lime milk.
Thus, the structure and the important properties of calcium hydroxide are discussed along with some of its uses. To learn more about this compound and other calcium compounds, such as CaCO3, register with BYJUS and download the mobile application on your smartphone.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Don’t Miss: Who Coined The Term Geography
Naturally Occurring Stable Nuclides
As noted, these number about 251. For a list, see the article . For a complete list noting which of the “stable” 251 nuclides may be in some respect unstable, see and . These questions do not impact the question of whether a nuclide is primordial, since all “nearly stable” nuclides, with half-lives longer than the age of the universe, are also primordial.
The Chemical Properties Of Calcium
Calcium is an active metal that enters into many interactions. In normal conditions, it easily reacts with the formation of corresponding binary compounds: with oxygen and halogens. Click here for learning more about calcium compounds. When heated it reacts with nitrogen, hydrogen, carbon, silicon, boron, phosphorous, sulfur and other substances. In open air, it immediately interacts with oxygen and carbon dioxide, so it becomes covered with a grey coating.It reacts violently with acids, sometimes bursting into flame. Calcium displays interesting properties in the composition of salts. For example, cave stalactites and stalagmites consist of calcium carbonate which gradually forms from water, carbon dioxide and hydrocarbonate under the influence of processes inside underground water.
Owing to its high activity in an ordinary state, calcium is stored in the laboratory in a dark glass, with a tightly closed lid and under a layer of paraffin or kerosene. The qualitative reaction to the calcium ion is that a flame turns a bright, rich brick-red color.
The metal can be identified in the composition of compounds by the undissolved sediments of some salts of the element
Read Also: What Is Q2 In Math
Ca2+ Signaling And Storage: The Endoplasmic Recticulum
The endoplasmic recticulum is generally the predominant intracellular Ca2+ store. The ERis critical not only for Ca2+ storage, but release of Ca2+ from the ER is responsible for rapidtransmission of Ca2+ signals from the periphery to the center of the cell and for local Ca2+signaling. Interestingly, changes in ER Ca2+ handling is thought to be involved in neural ageing and improper ER Ca2+ control has pathogenic implications. Resting ER free Ca2+ concentration is thought to be on the order of several hundred M, in addition, lumenal ERCa2+ is stored by high capacity-low affinity Ca2+ buffering proteins including calsequestrin andcalreticulin.
This resting level of lumenal ER free Ca2+ is the result of a balance between Ca2+ uptake via sarcoplasmic-endoplasmic type ATPases and release. Ca2+ is released from the ER in either a stimulated or passive manner.
Passive release is less well-defined and has been shown in many cell types to be remarkablydynamic as inhibition of the SERCA pump by thapsigargin often results in a rise in cytosolic free Ca2+ within a few seconds. This passive leak has been shown to be insensitive to inhibitors of both IP3 and RyRs, but responsive to ATP levels. Recent evidence has implicated a protein involved in apoptosis, Bcl2, as having a possible role in passive Ca2+ leak from the ER.
Ca2+ Evolution: A Rejection From Cytoplasm
An interesting theory on the evolution of the role of Ca2+ in biology has been presented byWilliams,, based on the ability for Ca2+ to precipitate organic anions. From life’s very beginnings, it was essential that Ca2+ be separated from organic anions to avoid precipitation. Thus,Ca2+ had to be rejected from the cytoplasm of the earliest cells. This initial simple rejection ofCa2+ is still observed for all organisms, ranging from prokaryotes to multicellular eukaryotes,where intracellular free Ca2+ concentrations are maintained on the order of 10-7M. In theearliest forms of life, namely prokaryotes, Ca2+ was simply rejected to intracellular levels on theorder of 10-5M. Little function for intracellular Ca2+ is found in anaerobic bacteria to this day.
Recommended Reading: What Is Diffusion In Geography
Interesting Facts About Calcium
- Calcium is the most abundant of the metallic elements in the human body. The average adult body contains about 1 kg or 2 lb of calcium, 99% of which is in the bones and teeth. Only oxygen, carbon, hydrogen and nitrogen are more abundant in our bodies than calcium.
- Calcium not only builds the structures that support our bodies, many of us also live in homes built using structural concrete or cement made with lime . Snails and many shellfish use another calcium compound calcium carbonate to build their own homes too their shells.
- Modern humans were not the first people to make use of calcium to build things. Egypts pyramids were built using limestone blocks. Limestone is crystalline calcium carbonate. In the later pyramids, the blocks were held together with gypsum or lime based mortar. Gypsum is calcium sulfate dihydrate and lime is calcium oxide.
- Have you ever wanted to be in the limelight? Lime is calcium oxide, which produces a brilliant, intense light when burnt in an oxyhydrogen flame. It was used to light the stage in theaters during the 1800s until electricity took over hence the saying.
- Cells in animals and plants must communicate with other cells. This is called signaling. Calcium ions are the most important messengers between cells in living things and are absolutely vital for the existence of multicellular life forms.
Commercial Production Of Calcium Carbonate
Calcium carbonate is produced commercially in two different grades. Both grades compete industrially based primarily on particle size and the characteristics imparted to a product.
PCC has smaller particles has a higher purity is less abrasive and tends to have higher brightness than GCC.
Also Check: What Are The 4 Major Goals Of Psychology
Channels That Lead To An Increase In Cytosolic Ca2+
Temporal and spatial control of increases in cytosolic free Ca2+ is of paramount importancefor proper functioning of most cells, and aberrant Ca2+ homeostasis can rapidly result in celldeath. Therefore, it is not that surprising that members of several different gene families of Ca2+ permeable channels may contribute to Ca2+ influx into the cytosol. The first members of most of these gene families were discovered in the eighties and nineties, quickly followed by cloning of related gene products by now with the human and mouse genomic sequencing projects nearing completion, a complete membership list is probably available for most genefamilies. Many of these channels contributing to Ca2+ influx into the cytosol can be foundcoexpressed in most cell types, especially in cells of excitable tissues. To reflect the diversity ofspecific cellular functions, most of the above gene families contain many different but relatedmembers, and this complexity is further increased by the presence of different splice variants.Although the occurrence of different splice variants is common, only in very few cases has a physiological significance been established with certainty.
Uses Of Calcium Hydroxide
- In the process of sewage treatment, calcium hydroxide is used as a clarifying agent or as a flocculant.
- Ca2 is used in the paper industry during the Kraft process of converting wood into wood pulp.
- It is a very important compound in the preparation of ammonia.
- This compound is also used as a pH modifier due to its basicity.
- The pickling of cucumbers is generally done with the help of Ca2
- The production of many plastics involves the use of calcium hydroxide as an ingredient.
- It is also used in pesticides, hair care products, and the manufacture of ebonite.
- In root canal procedures, this compound is used to fill the cavities in the human teeth.
- Sugar beets and sugarcane are processed via carbonation, which involves the use of Ca2.
- Calcium hydroxide is used in the leather industry to separate the fur/hair from the animal hide.
Also Check: What Is Spatial Science In Geography
How To Write The Electron Configuration For Calcium
In order to write the Calcium electron configuration we first need to know the number of electrons for the Ca atom . When we write the configuration we’ll put all 20 electrons in orbitals around the nucleus of the Calcium atom.
In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We’ll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we’ll move to the 3p where we’ll place the next six electrons. We now shift to the 4s orbital where we place the remaining two electrons. Therefore the Calcium electron configuration will be 1s22s22p63s23p64s2.
Video: Calcium Electron Configuration Notation
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Discovery Of The Element Calcium
Elemental calcium metal was first isolated in 1808. Humphry Davy isolated calcium after discovering several other elements which included potassium and sodium. He isolated calcium through electrolysis, a technique he has also used to isolate other elements. Electrolysis of lime and mercuric oxide was performed to obtain calcium. After the electrolysis, he used distillation to purify the calcium.
You May Like: Kuta Software Infinite Geometry Similar Triangles
Intracellular Ca2+ Release Channels
Two families of intracellular Ca2+ release channels have been found to date, the IP3 receptor and the Ryanodine receptor , each family consisting of three distinct mammaliangenes. The IP3 and Ryanodine receptors are among the largest ion channel proteins and formfunctional tetramers with a MW of about 1.2 and 2 million daltons, respectively.
Properties Of Calcium Oxide
- Quick lime is an amorphous white solid with a high melting point of 2600°
- It is a very stable compound and withstands high temperatures.
- In the presence of water, it forms slaked lime. This process is called the slaking of lime.
CaO+H2O Ca 2
- It is an oxide that is basic in nature and forms salts when it comes in contact with an acid.
- This compound crystallizes in a cubic crystal lattice.
- The standard molar entropy associated with calcium oxide corresponds to 40 joules per mole kelvin.
- This compound is known to emit an intense glow when it is heated to temperatures above 2400 degrees celsius.
CaO+H2SO4 CaSO4+H2O
- It is extensively used for medicinal purposes and insecticides.
- It finds its application in the manufacturing of cement, paper, and high-grade steel.
- Lime is used as a reagent in laboratories for dehydration, precipitation reaction, etc.
- It is the cheapest alkali available which is an important ingredient in the manufacturing of caustic soda.
- Calcium is essential to animal life as the constituent of bones, shells, and teeth. The most common of the calcium compounds are calcium carbonate which the potter uses as a source of calcium oxide for glazes.
You May Like: What Is Electronegativity In Chemistry
The Reaction Of Water With Calcium
Calcium is stored in container under a layer of protective liquid. To conduct an experiment to demonstrate how the reaction of water and calcium takes place, you cannot just take out the metal and cut off a piece of it. It is easier to use metallic calcium in the laboratory in the form of filings which are prepared with a lathe.
If you dont have any filings, and there are no small pieces of calcium in the container, then you will need pliers or a hammer. Use the tool to separate the required piece of calcium, and place it in a flask or a glass of water. A calcium filing is placed in a dish in a gauze bag.
The calcium sinks to the bottom, and hydrogen is released firstly in the place where the metal was broken off. Gradually, gas is released from the surface of the calcium the process resembles vigorous boiling. At the same time a sediment of calcium hydroxide is formed.
What Is The Formula For Calcium Carbonate
The formula for calcium carbonate is CaCO3.
Learn more about Calcium Oxide, Calcium Sulphate and more with BYJUS by downloading our app.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
You May Like: What Is C5 In Chemistry
Ca2+ Chemistry Storage And Transport In Biologic Systems: An Overview
Tashi G. Kinjo and Paul P.M. Schnetkamp.
Calcium ions play a critical role in most if not all cellular processes. It has even beendemonstrated that Ca2+ currents in root tips, in combination with gravity, areresponsible for their downward growth. Most of these effects are mediated by both temporally and spatially tightly controlled changes in cytosolic free Ca2+ brought about byactivation of Ca2+ influx pathways in the cell membrane or by activation of intracellular Ca2+release channels, and countered by transporters acting as Ca2+ pumps. Voltage-gated Ca2+ channelsare a preeminent class of plasma membrane proteins providing regulated Ca2+ influx, and the remainder of the volume is dedicated to their many different facets. In this introductory chapter, we will briefly review other aspects of cellular Ca2+ homeostasis. We will review some of the chemical properties of Ca2+ that are important to its role in biology, we will review intracellular Ca2+ stores, and we will review other Ca2+ handling proteins. Due to space limitations we will not be able to refer to the original literature in most cases, but have to limit ourselves to a relatively small number of recent reviews that could provide the reader further guidance into the many exciting and sometimes controversial topics of current research in Ca2+ transport and storage in cells.