Examples Of End Point In A Sentence
end point The New Republicend pointUSA TODAYend pointOutside Onlineend point ajcend point Fox Newsend pointOutside Onlineend point The Salt Lake Tribuneend pointchicagotribune.com
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘end point.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
What Is Kinetic Reaction
Kinetic reaction method is an analysis method used in clinical chemistry to determine the difference in absorbance between two points of the progression of a reaction. Here, we use a specified time period for this determination. We should assume this determination a constant amount of product forms during the time interval being monitored. Usually, we consider a short time period . That is to avoid any effect comes from enzyme degradation during the progression of the reaction.
Before starting the reaction, we should perform a pre-incubation in order to avoid any interferences comes from substances other than the analyte. During the pre-incubation, these substances completely react with the reagent system. There are two major types as
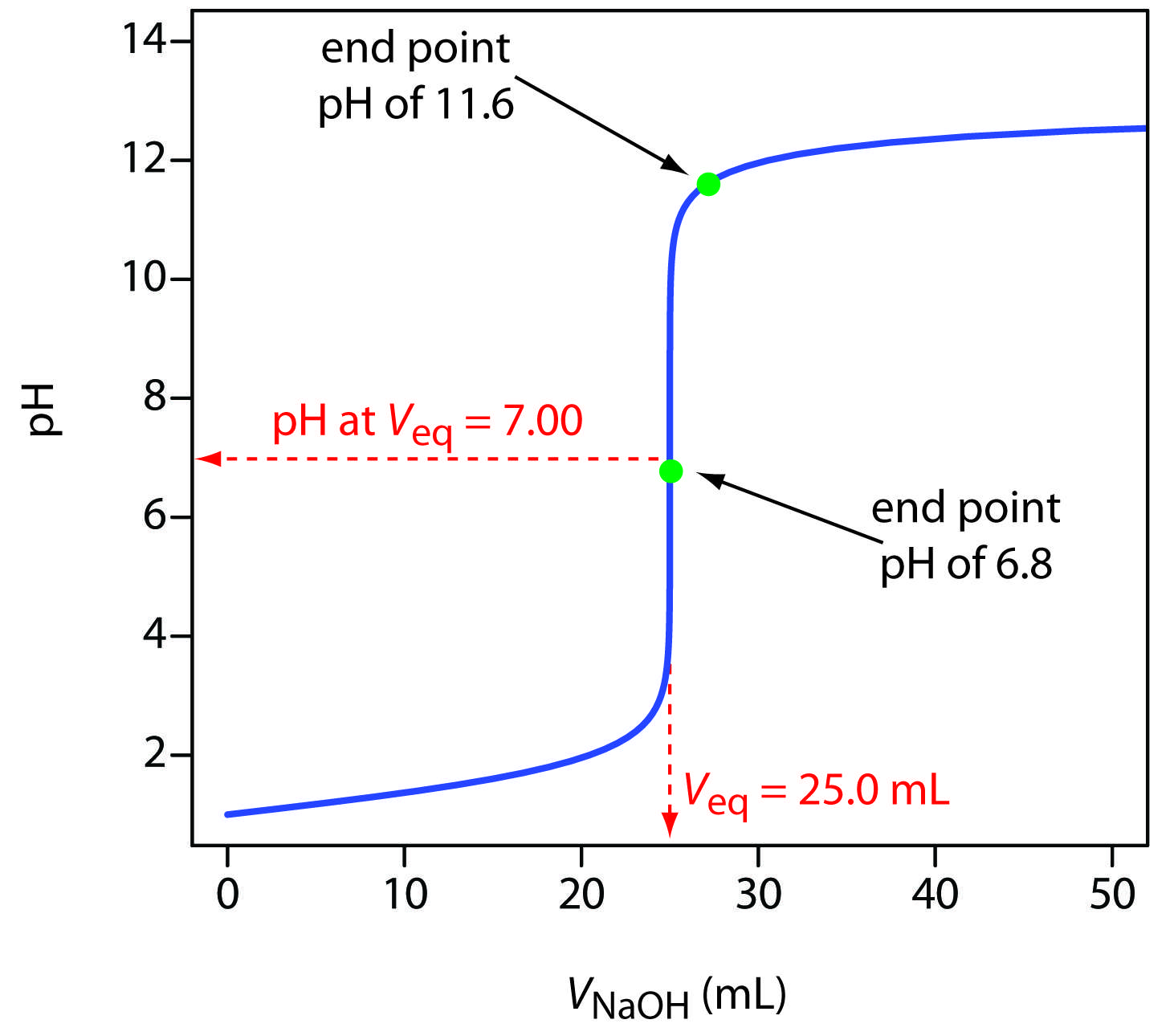
Endpoint Vs Equivalence Point
| Endpoint | Equivalence point |
| The point at which color change i.e. occurs in the system due to pH change is called endpoint | At equivalence point, the chemical reaction in the titration mixture ends |
| At this point, the moles of titrant exceed the moles of the analyte. A sharp change in pH occurs at this point resulting in color change of the indicator | It is the exact point in a titration when the number of moles of titrant are equal to the number of moles of analyte |
| It comes after equivalence point | It occurs before the endpoint |
| Titration is complete once the endpoint reaches | It does not indicate the completion of a titration process |
| Endpoint does not mean the completion of reaction between analyte and titrant | Equivalence point means the completion of reaction between titrant and analyte |
| It occurs once in a reaction | A titration process can have multiple equivalence points |
| Change in color | Just before change in color |
You May Like: What Is Bilateral Symmetry In Biology
Endpoint Vs Equivalence Point: Whats The Main Difference
Endpoint and equivalence points are often confused. Both are important stages of any titration experiment and have many differences. Like other titration terms such as titrants, analyte, burette, and pipette, endpoint and equivalence points are equally important in fully understanding the titration technique.
In a nutshell,
Endpoint is the point in the titration process where the indicator changes its color whereas the equivalence point indicates the completion of the reaction between titrant and the substance being titrated .
Applications Of Titration In Todays World
This analytical technique has many applications, ranging from medical, to cosmetic, and even to environmental science.
In the food industry, workers use titration and equivalence points to determine the quantity of different salts, sugars, vitamins, and fatty acids in foods. Similarly, other major industries like cosmetics and cleaning use titration to add appropriate and safe concentrations of chemicals to their products. Pharmaceutical companies rely on titration during the medication developing process. Even medical labs use this type of analysis to study blood and urine samples from patients.
In addition to industry, there are many uses for titration in academic research. For one, environmental chemists conduct titrations using rainwater, melted snow, and other liquids to assess their composition. For analysis of water content in an analyte, there is a special technique known as Karl-Fischer titration. In these and many other fields, titration also serves to determine the exact concentration of reagents, a process known as standardization.
As you can see, titration has an astounding range of uses and applications. As a result, it is one of the most frequently conducted methods of chemical analysis worldwide, both in the classroom and in the workplace.
Recommended Reading: What Are Grid Lines In Geography
What Should You Know About The Endpoint Of A Chemical Reaction
The endpoint in a chemical reaction is the point where it changes color during the titration process. It represents the end of the titration.
We can attain an endpoint by carefully handling the number of drops of the titrant. We can change the PH of a solution by a single drop. The endpoint is also known as a volumetric point.
Meaning And Definition Of Endpoint :
the point in a titration at which the indicator changes color.
For the term endpoint may also exist other definitions and meanings, the meaning and definition indicated above are indicative not be used for medical and legal or special purposes.
Source : SFU Text file : http://school.gogpg.com/Portals/1/Assess%20Well/Example%20Sampling%20Domains-Curriculum%20Specific.xls
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship please send us an e-mail and we will remove your text quickly.
Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author’s work under a four-factor balancing test.
Read Also: What Does Trajectory Mean In Physics
What Is The Endpoint Of Titration Called
The point in the titration process where the chemical reaction in the titration mixture ends is called the equivalence point. The point in the titration process which is indicated by the colour change of the indicator is called the endpoint. 2. It is the point where the analyte has completely reacted with the titrant.
S Of Finding The Equivalence Point
There are several different ways to identify the equivalence point of a titration:
Color Change – Some reactions naturally change color at the equivalence point. This may be seen in redox titration, particularly involving transition metals, where the oxidation states have different colors.
pH Indicator – A colored pH indicator may be used, which changes color according to pH. The indicator dye is added at the beginning of the titration. The color change at the endpoint is an approximation of the equivalence point.
Precipitation – If an insoluble precipitate forms as a result of the reaction, it can be used to determine the equivalence point. For example, the silver cation and chloride anion react to form silver chloride, which is insoluble in water. However, it can be difficult to determine precipitation because the particle size, color, and sedimentation rate may make it difficult to see.
Conductance – Ions affect the electrical conductivity of a solution, so when they react with each other, the conductivity changes. Conductance may be a difficult method to use, especially if other ions are present in the solution that can contribute to its conductivity. Conductance is used for some acid-base reactions.
Spectroscopy – Spectroscopy can be used to find the equivalence point if the spectrum of the reactant, product, or titrant is known. This method is used to detect etching of semiconductors.
Recommended Reading: Common Core Math 7 Eog Questions Geometry
What Should You Know About The Equivalence Point In A Chemical Reaction
To understand the definition of the Equivalence point, you should know that it is the actual point in a titration where the moles of one titrant get equaled to the moles of the other substance being titrated. This point is known as the equivalence point.
For instance, in an acid-base titration, the moles of the base will get equaled to the moles of the acid at the equivalence point. As the titration advances, we use the change in pH, to monitor acid-base titrations. The equivalency point is not anything like the endpoint of the titration process.
Do you know the methods to determine the equivalence point?
Well, that is not difficult at all. The method includes PH change, color change, the difference in conductivity, change in temperature, and formation of a precipitate. We can find the equivalence or stoichiometric point in a titration process when there is enough base and acid to neutralize the solution.
Do you know?The Equivalence point in a chemical reaction is also known as the Stoichiometric point.
S For Determining The Equivalence Point
- Color change of self- indicators – In the case of reactions with self-indicators as reactants, the color change shows the equivalence point of the titration, since no indicators are used.
- Endpoint – Sometimes equivalence points can be considered an endpoint because they are roughly the same.
- Conductance – The conductance can also be used to determine the equivalence point of the titration. Here the conductance should be measured during the entire titration, and the equivalence point is where a rapid change in conductance occurs. This is a somewhat difficult method.
- Spectroscopy – This method can be used for colorful reaction mixtures. The determination is based on the rapid change in the wavelengths absorbed by the sample.
Recommended Reading: How To Study Geography Maps
What Is The Difference Between Kinetic And End Point Reaction
Kinetic reaction method is an analysis method used in clinical chemistry to determine the difference in absorbance between two points of the progression of a reaction. Here, we measure the difference in absorbance between two points during the progression of the reaction. Moreover, the time taken for this reaction is around 20 seconds to 1 minute. Whereas, the end point reaction method is an analysis method used in clinical chemistry to determine the total amount of analytes consumed during the progression of a reaction. In this method we measure the total amount of analytes that participate in the reaction. Furthermore, the time period taken for this reaction is 5 to 15 minutes.
Difference Between Endpoint And Equivalence Point
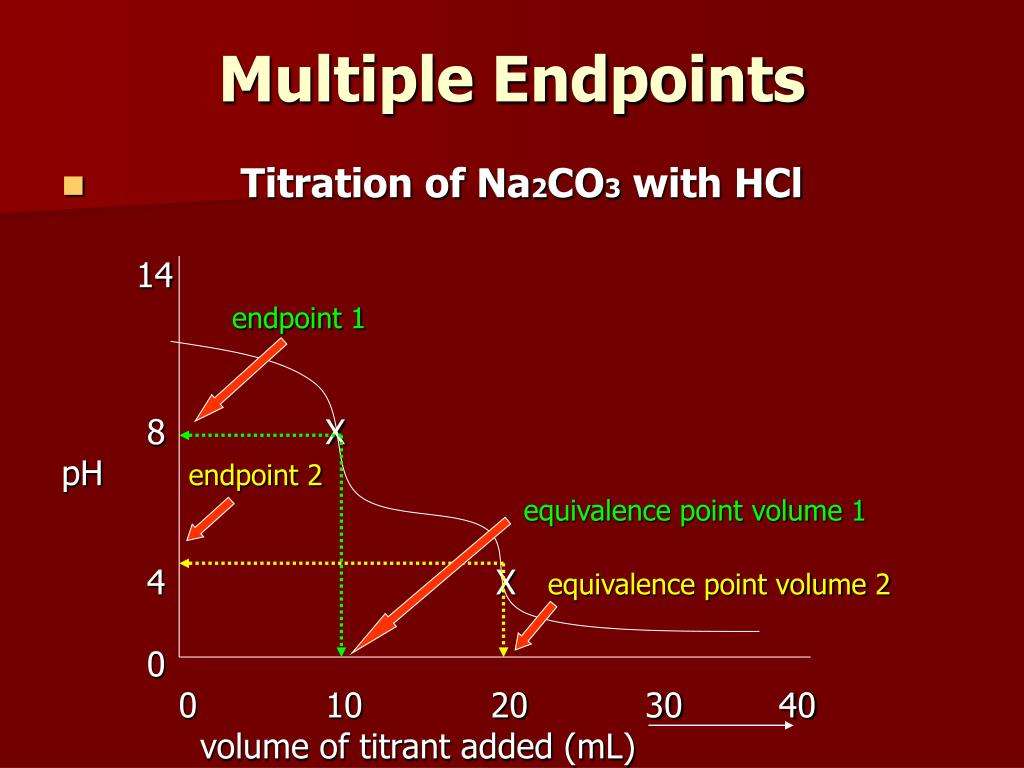
Titration is used in analytical chemistry to determine acid, bases, reductants, oxidants and other species. Titrations can usually occur in reactions such as redox reactions and acid-base reactions. During the process, two important stages known as endpoint and equivalence point are reached. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. On the other side, Endpoint is a point where the solution changes colour. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end while the endpoint is the point where the colour change occurs in a system.
Don’t Miss: What Does Additive Mean In Math
Definition Of Endpoint Titration
Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The ideal point for the completion of titration is known as the equivalence point. The end point demonstrates the equivalence point, typically by some form of indicator. For example, with a color indicator, the solution changes color when the titration reaches its end point.
TL DR
The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The end point typically comes straight after the equivalence point, which is when the moles of a standard solution equal the moles of a solution of unknown concentration , i.e., the ideal point for the completion of titration. In a perfect titration, the end point and equivalence are identical.
How To Perform A Simple Titration
The following steps describe the methods involved in a titration analysis using a visual indicator:
Also Check: What Are The Best Colleges For Psychology
What Is The Definition Of A Chemical Reaction
It is a reaction in which a chemical change occurs while combining two or more substances and forming a new material. A chemical reaction regroups the basic atoms of the reactants, which results in the formation of various substances as products. The products have features that are distinct from the reactants.
These reactions are fundamental aspects of technology, society, and even existence. Many activities involving chemical changes that have been recognized and practiced for thousands of years include heating fuels, smelting iron, creating glass and pottery, making beer, and manufacturing wine and cheese.
The examples are:
- We mix Iron and Sulphur to form Iron Sulphide
Fe + S FeS
- We can make slaked lime by combining Calcium Oxide and water . The reaction will be,
Cao + H2O Ca 2
- Electrolysis is an endothermic activity that breaks down water into its constituent atoms. We complete this process using electrical energy rather than thermal energy. The reaction will be.
2 H2O 2 H2 + O2
S Of Determining The Equivalence Point
- Color change of self-indicators In reactions involving self-indicators as reactants, the color change indicates the equivalence point of the titration since indicators are not used.
- Endpoint Sometimes, equivalence point can be considered as the endpoint since they are approximately equal.
- Conductance Conductance can also be used to determine the equivalence point of the titration. Here, the conductance should be measured throughout the titration, and the equivalence point is where a rapid change of conductance occurs. This is a bit difficult method.
- Spectroscopy This method can be used for colorful reaction mixtures. The determination is done according to the rapid change in wavelengths that are absorbed by the sample.
Recommended Reading: What Do You Learn Psychology
Titration Technique Of Analytical Chemistry
Titration is a technique used in analytical chemistry to determine the concentration of unknown solutions by using solutions of known concentration. Solution of known concentration is known as titrant while the solution of unknown concentration is known as analyte in titration technique.
As we all know, the number of diabetes patients is increasing day by day worldwide. Do you know the drugs used for the treatment of diabetes contain metals in a specific amount? The metal content in a drug can be determined by titration techniques . It is a very useful, simple, and low-cost technique for various medicinal applications in the pharmaceutical field. So, you should have a good understanding of the titration technique.
To understand the titration technique, you need to have a clear understanding of the terms related to it such as a pipette, burette, titrant, analyte endpoint and equivalence point, etc. Generally, students get confused between endpoint and equivalence point so, in this article, we will discuss these two terms clearly and comparatively in detail.
Endpoint and equivalence points are closely related and confusable. Both the points show very important stages of titration during performing the titration experiment. Still, both points are different and show two different stages of titration.
Main Differences Between Endpoint And Equivalence Point
Also Check: How Does Chemistry Relate To Cancer
Difference Between Endpoint And Equivalence Point Definition Examples
Endpoint and equivalence point are the terms most commonly used in chemistry titrations. Most of the time, these terms are used interchangeably and thought to be the same. Endpoint and equivalence point are two different concepts seen that come across as titrations. This article deals with explaining the concepts of endpoint and equivalence point along with elucidating the key differences between the both.
Before we move on to the point, let us recall a little bit about titrations. Titration may be defined as the volumetric analysis of the given sample by the addition of a known quantity of another substance called the titrant. Titration is usually performed to determine the unknown concentration of the given sample or analyte. Endpoint and equivalence points are basically two different stages in the titration process. An equivalence point is a stage where the total number of moles of two solutions become equal. It means that the amount of titrant added is chemically equivalent to the given sample. The endpoint follows the equivalent point. An endpoint represents a stage where it signals the completion of the reaction with a change in colour or intensity.
Endpoints and equivalence points are widely observed in acid-base reactions. Let us study a little deeper about both of them to get a clearer picture.