Surface Area Of Solid Reactants
Increasing the surface area of reactants frees up more particles that are available to collide right away. This doesn’t add more particles in to the reaction, it only makes available particles that would have once waited for other particles to react first until they could be available. This leads to more frequent, successful collisions and so the rate of reaction increases.
C52 Displacement Reactions Aqa Gcse Chemistry C5 Chemical Changes Kerboodle Answers : Page No 87
In the reaction between iron and zinc sulphate
The reaction will not take place as iron is less reactive than zinc so it will not displace zinc from zinc sulfate.
Reaction between zinc and copper sulphate will take place as Zinc is more reactive than copper so it will displace copper from its solution. Zinc displaces copper from copper sulfate& forms zinc sulfate solution. This is indicated by color change from blue to colorless. CuSO4solutionhas a blue color while ZnSO4solutionis colorless. Zn + CuSO4 -> Cu + ZnSO4 .
Reaction between magnesium and iron chloride will take place as magnesium being more reactive than iron displaces iron from iron chloride and forms magnesium chloride. Magnesium reacts with iron chloride to form magnesium chloride displacing iron.
3Mg + 2FeCl3 => 3MgCl2 +2Fe
Carbon is higher in the reactivity series than zinc but is lower in the reactive series than magnesium. Carbon being more reactive than zinc can displace zinc from its oxide and form zinc and carbon dioxide. However, carbon cannot reduce magnesium from its oxide as it is less reactive than magnesium so it cannot displace magnesium. Therefore, magnesium can only be extracted by electrolysis.
Tungsten is less reactive than hydrogen and hence is displaced by hydrogen in tungsten oxide to form tungsten and water.
Zn + Fe2+ Zn2+ + Fe
Zn = Zn2+ + 2e-
Fe2+ +2e- = Fe
Moles of Zinc = 3.25/65 = 0.05 moles
Zn + FeSO4 ZnSO4 + Fe
C: Monitoring And Controlling Chemical Reactions
More in this topic
Chemical reactions take place when the reactant particles collide together, and then form new products.
Not every collision is successful. The particles have to collide:
- with the right orientation
- with the right amount of energy
Rates of reaction are increased when the frequency and/or the energy of collisions is increased.
Also Check: Explain Why There Are Different Branches Of Chemistry
C 56 Making More Salts Aqa Gcse Chemistry C5 Chemical Changes Kerboodle Answers: Page No 95
Acid + base salt + water.
H+ and OH- reacts to form water.
H+ + OH- = H2O .
Acid and the metal carbonate react to form salt, water and carbon dioxide.
Acid + metal carbonate salt + water + carbon dioxide.
Lithium chloride is produced by the treatment of lithium carbonate and hydrochloric acid.Lithium chloride is produced by the treatment of
lithium carbonate and hydrochloric acid. Lithium
carbonate is soluble. We will first titrate the
lithium carbonate with hydrochloric acid in the
presence of indicator to know the exact volume of
lithium carbonate and hydrochloric acid that will
react completely in a neutralisation reaction.
When the exact volume is known the same reaction
will be carried out but this time without the
indicator. After the reaction lithium chloride,
carbon dioxide and water will be formed. Carbon
dioxide will escape and the aqueous solution of
lithium chloride will be evaporated to evaporate
the water and lithium chloride crystals will be
collected.
Lithium carbonate reacts with hydrochloric acid to form lithium chloride, carbon dioxide and water
Li2CO3 + 2HCl > 2LiCl + CO2 + H20.
Barium carbonate react with nitric acid to form barium nitrate, water and carbon dioxide.
BaCO3 + 2HNO3 > Ba2+ + 2 + CO2 + H2O
When acids and metal carbonates react, hydrogen ions and carbonate ions react to form water and carbon dioxide.
Banner 7
Selective Terminal Cc Scission Of C5
F. van der Klis, L. Gootjes, J. van Haveren, D. S. van Es and J. H. Bitter,Green Chem., 2015, 17, 3900DOI: 10.1039/C5GC01012H
This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence. You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes.
To request permission to reproduce material from this article in a commercial publication, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party commercial publication please go to the Copyright Clearance Center request page.
Read Also: What Does Commensalism Mean In Biology
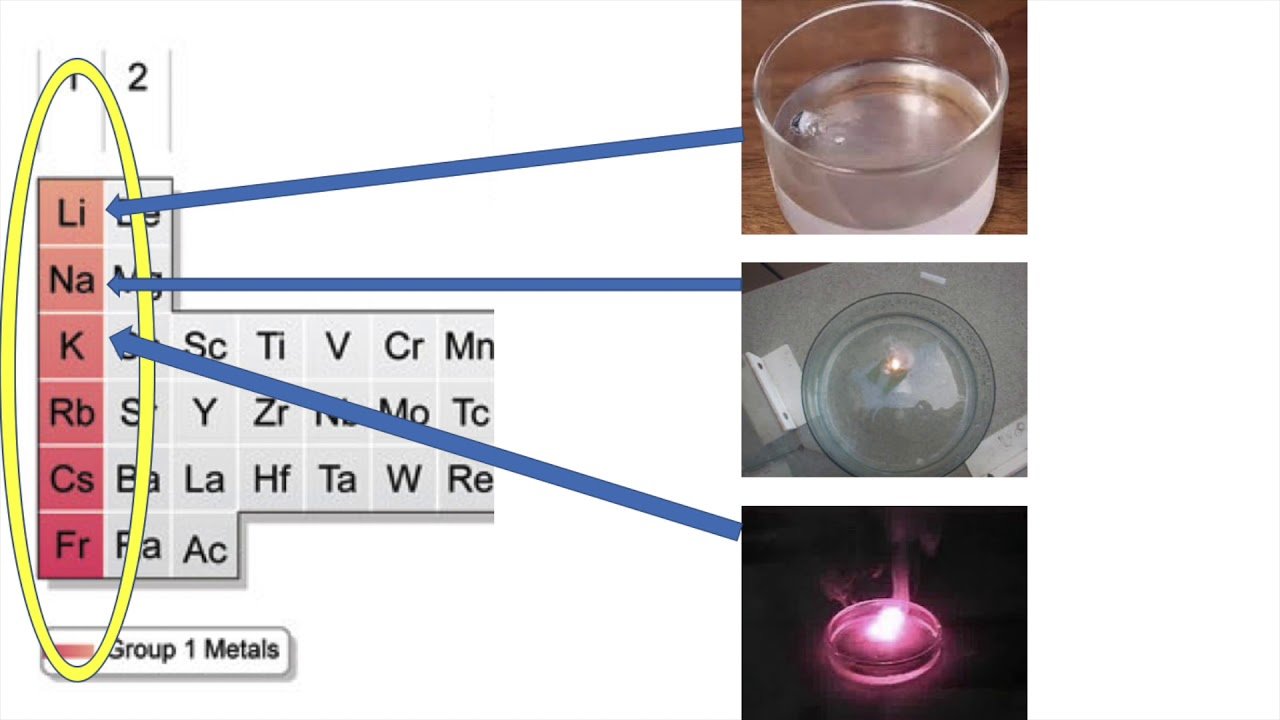
C 51 The Reactivity Series Aqa Gcse Chemistry C5 Chemical Changes Kerboodle Answers : Page No 85
Lithium + water lithium hydroxide + hydrogen
2Li + H2O = 2LiOH + H2
Zinc + Hydrochloric acid Zinc chloride + Hydrogen
Zn + 2HCl ZnCl2 + H2
When the magnesium ribbon reacts with dilute sulphuric acid, we can see the following observation
Bubbles of hydrogen gas will be released. Magnesium ribbon will dissolve to form magnesium sulfate. The test tube will also become warm as the reaction is exothermic.
Mg + H2SO4 = MgSO4 + H2
Magnesium + Sulphuric Acid = Magnesium sulphate + hydrogen.
These metals placed are found at the bottom of the reactivity series. Hence, they are very unreactive. Gold and platinum are even known as noble metals. They are not affected by air, water and even by chemicals. Since they have bright lustre and resistant to reaction with air, water and chemicals which makes them useful to make jewellery.
Potassium, lithium, sodium are stored in oil as they all react with water producing lots of heat. As a result, hydrogen evolved catches fire. They cannot be kept in air also because air contains moisture or water vapour. These are kept under kerosene to avoid contact with both air and water.
Zinc is more reactive than tin. Which may react with food items and make it unfit for health. Therefore, food cans are plated with tin but no zinc.
4Fe + 3O2 = 2Fe2O3
Banner 2
C55 Salts From Insoluble Bases Aqa Gcse Chemistry C5 Chemical Changes Kerboodle Answers : Page No 93
Acid + alkali salt + water.
Aluminium Sulphate = Al23
Take a clean beaker and put the powdered impure sample of copper sulphate in it. Add distilled water and stir the contents gently with the help of a glass rod. In order to make the solution more clear add two or three drops of concentrated sulphuric acid in it. Heat the solution in the beaker to 60-700C on a wire gauze. Stir it continuously and add more impure copper sulphate until no more of it dissolves. Filter the solution and collect the filtrate in a china dish. Place the china dish over wire gauze kept over a tripod stand and heat it gently .
As the solution gets heated, stir it with a glass rod.
This helps in uniform evaporation and prevents the formation of a solid crust. When the volume of the solution reduces to one-half, take out a drop of the concentrated solution on one end of the glass rod and cool it by blowing air. Formation of thin crust indicates that it reaches to the crystallization point. Turn off the burner, cover the dish with a watch glass, and keep it undisturbed. As the solution cools down, crystals separate out. Slow cooling ensures better crystallization. Decant the mother liquor and wash the crystals with a thin stream of cold water with the help of a wash bottle. Dry the crystals by pressing them gently between sheets of filter paper.
Li2O + H2SO4 = Li2SO4 + H2O .
You May Like: Is Paris Jackson Michaels Biological Daughter
C54 Salts From Metals Aqa Gcse Chemistry C5 Chemical Changes Kerboodle Answers : Page No 91
metal + acid salt + hydrogen
Copper sulphate cannot be prepared by adding copper to sulphuric acid because copper is not an active metal its activity is less than that of hydrogen in the activity series so it cannot displace hydrogen from the acid and form a salt.
Potassium is never prepared by reacting potassium with hydrochloric acid because the reaction between potassium and hydrochloric acid is very violent, even explosive. On contact with the acid the reaction rapidly releases heat and hydrogen.
Fe + H2SO4 = FeSO4 + H2 .
Zn + 2HCl = ZnCl2 + H2.
Zn + 2 H+ Zn2+ + H2.
: Zn = Zn2+ + 2e-
Reduction half equation in which hydrogen ion gains two electrons.
: 2H+ + 2e- = H2.
Reaction between Zinc and hydrochloric acid is a redox reaction as In this reaction, zinc is getting oxidised as it is losing electrons and hydrogen is getting reduced as it is gaining electrons. Since both oxidation and reduction taking place in the same reaction, it is a redox reaction.
Banner 5
Nhx Interactions Stabilize Intra
V. R. Mundlapati, Z. Imani, V. C. D’mello, V. Brenner, E. Gloaguen, J. Baltaze, S. Robin, M. Mons and D. J. Aitken,Chem. Sci., 2021, 12, 14826DOI: 10.1039/D1SC05014A
This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence. You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes.
To request permission to reproduce material from this article in a commercial publication, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party commercial publication please go to the Copyright Clearance Center request page.
Read Also: College Algebra 6th Edition Dugopolski Pdf
What Is C1 C2 C3
. Herein, what is c1 c2 c3 c4 c5 gas?
THE LIGHT HYDROCARBONS-methane , ethane , propane , and the butanes , either in the gas phase or liquefied, are primarily used for heating, motor fuels, and as feedstocks for chemical processing.
Beside above, what is c1 c2 c3 in Canon 7d? Canon 7D: Camera User Settings C1 C2 C3. The Canon 7D has the capability of storing all of your settings into one shortcut, the C1, the C2 or the C3. It will store all of the settings from the Q , the Quick Control Screen, the menus, the custom functions
Also know, what is c2 c3 and c4?
The C2-C5 spinal motion segments comprise three individual segments: C2–C3, C3–C4, and C4-C5. This group of motion segments starts with the C2 vertebra near the top of the cervical spine and ends in the mid-cervical spine at the C5 vertebra.
What does c3 mean in school?
C1, C2, C3 Attendance is taken by each class.
Chapter 1 Analysis Of C5 And Lighter Hydrocarbons
THE LIGHT HYDROCARBONS-methane , ethane , propane , and the butanes , either in the gas phase or liquefied, are primarily used for heating, motor fuels, and as feedstocks for chemical processing. The pentanes/pentenes are products of natural gas or petroleum fractionation or refinery operations that are removed for use as chemical feedstocks. The olefins-ethene , propene , butenes , pentenes, and pentadienes are materials produced by various refining processes involving the use of the saturated hydrocarbons as feedstocks.
Recommended Reading: Geometry Angle Addition Worksheet
C53 Extracting Metals Aqa Gcse Chemistry C5 Chemical Changes Kerboodle Answers: Page No 89
Ores are naturally occurring rocks that contain metal or metal compounds in sufficient amounts to make it worthwhile extracting them. The method used to extract a given metal from its ore depends upon the reactivity of the metal.
Gold is a very inert metal, it doesnt readily react with other things, so it is not found chemically combined with other materials. Silver is less inert than gold, sometimes it is found in the metallic state, but often as compounds of silver such as, silver chloride, etc.
Since platinum is found in the native state, it means platinum is unreactive and is placed lower in the reactivity series. As it is noble metal and unreactive it will maintain its metallic luster and is best suited for making jewelry.
Zinc oxide + Carbon = Zinc + Carbon Monoxide
Zinc is getting reduced and carbon is getting oxidised.
ZnO + C = Zn + CO .
Banner 4
Practice Questions Aqa Gcse Chemistry C5 Chemical Changes Kerboodle Answers: Page No 101
01.1. Answer.
The one that produce highest fizzing is the most reactive and the one that has no fizzing is least reactive so the order is :
B D-C-E-A
01.2. Answer.
The test for hydrogen is a squeaky pop test. When a split is brought near to the mouth of hydrogen gas it burns with a squeaky pop.
lit splint pop.
01.3. Answer.
The variable that needs to be controlled in the experiment are :- same amount of metal and the similar temperature of water in all the test tubes.
01.4. Answer. A2
A is group 2 so will have +2 charge and OH- has a charge of -1 so the formula will be A2
2.1 Copper is less reactive than hydrogen so cannot displace hydrogen from acid therefore copper metal does not react with dilute sulphuric acid.
02.2. Answer.
The student heated the sulphuric acid To increase the rate of reaction as increase in temperature increases the rate of reaction.
02.3. Answer.
The acidic colour can either be red, orange or yellow.
02.4. Answer.
To ensure all the acid is used up we should add copper oxide is excess.
03.1. Answer.
When iron reacts with copper sulphate solution, iron will displaced copper so brown colour of copper will be formed and blue coloured copper sulphate solution will change into pale green iron sulphate.
03.2. Answer.
When magnesium reacts with iron sulphate, magnesium displaced iron and forms magnesium sulphate. Pale green solution of iron sulphate will change to colourless solution of magnesium sulphate.
03.3. Answer.
03.4. Answer.
gained electrons.
Read Also: Geometry Segment Addition Postulate Worksheet
Aqa Gcse C3 C4 C5 C6 C7 Sequence Of Lessons
Head of Science and examiner for AQA and OCR.
Your rating is required to reflect your happiness.
It’s good to leave some feedback.
Something went wrong, please try again later.
This resource hasn’t been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Recommended Reading: Algebra 1 Age Word Problems
C58 Strong And Weak Acids Aqa Gcse Chemistry C5 Chemical Changes Kerboodle Answers: Page No 99
The beaker with the ethanoic acid will have a higher pH and the beaker with nitric acid will have a lower pH.
pH= 5
Neutral Solution has a pH of 7 so the H+ Concentration will be 1.0X10^-7 mol/dm3.
Propanoic Acid is a weak acid is it partially dissociated to give H+ions when dissolved in water. On the other hand, nitric acid is completely dissociated to give H+ ions therefore is considered as a strong acid.
4.Answer.
The pH is a measure of the number of hydrogen ions in solution. The strength of an acid depends on the concentration of the solution and whether or not it fully ionizes or partially ionizes in water. A Strong acid will be completely dissociated to give the H+ ions and will be diluted which will neutralize the H+ to give the higher pH value. On the other hand, the weak acid being concentrated will not dissociate to give enough H+ ions that can give a high pH value.
Banner 9
Demand From Different Sectors Is Expected To Rise Resulting In Gasoline
C5 C8 n-paraffin fractions are further processed and separated to form several chemical products such as n-pentane, n-hexane, and n-heptane, among several other chemical compounds. The demand for n-pentane for the production of polystyrene foam and pharmaceuticals is growing which in turn is fueling the growth of n-pentane in the market.
Furthermore, the swelling demand for n-Hexane from end-use industries such as adhesive and sealant, agrochemical, and pharmaceutical, among others is anticipated to drive the demand for n-hexane and subsequently benefit the C5-C8 normal paraffin market over the course of the forecast period. N-Heptane is employed various synthesis and adhesive and sealant applications.
Demand for adhesives and sealants is intensifying owing to the intensive progress in automobile production and surging demand from packaging materials, which is likely to fuel the growth of the n-heptane market and, in turn, positively impact the C5-C8 n-paraffin market. N-octane n-paraffin compounds are most commonly employed in the oil and gas industry for analyzing the octane number in automobile fuel as well as many other gasoline and petrochemical products.
Additionally, rising demand for gasoline and petrochemical products is anticipated to stem from plastic, adhesive and sealant, pharmaceutical, chemical, and cosmetic industries, which in turn is expected to bolster the growth of the global C5 C8 n-paraffin market in the coming years.
Customize this Report
Also Check: Who Are The Biological Parents Of Prince Paris And Blanket