What If B Is Slightly More Electronegative Than A
B will attract the electron pair rather more than A does.
That means that the B end of the bond has more than its fair share of electron density and so becomes slightly negative. At the same time, the A end becomes slightly positive. In the diagram, “\” means “slightly” – so \ means “slightly positive”.
A polar bond is a covalent bond in which there is a separation of charge between one end and the other – in other words in which one end is slightly positive and the other slightly negative. Examples include most covalent bonds. The hydrogen-chlorine bond in HCl or the hydrogen-oxygen bonds in water are typical.
If B is a lot more electronegative than A, then the electron pair is dragged right over to B’s end of the bond. To all intents and purposes, A has lost control of its electron, and B has complete control over both electrons. Ions have been formed. The bond is then an ionic bond rather than a covalent bond.
Electronegativity Definition What Is Electronegativity
The definition of electronegativity is:
The tendency of an atom to attract electrons to form a chemical bond.
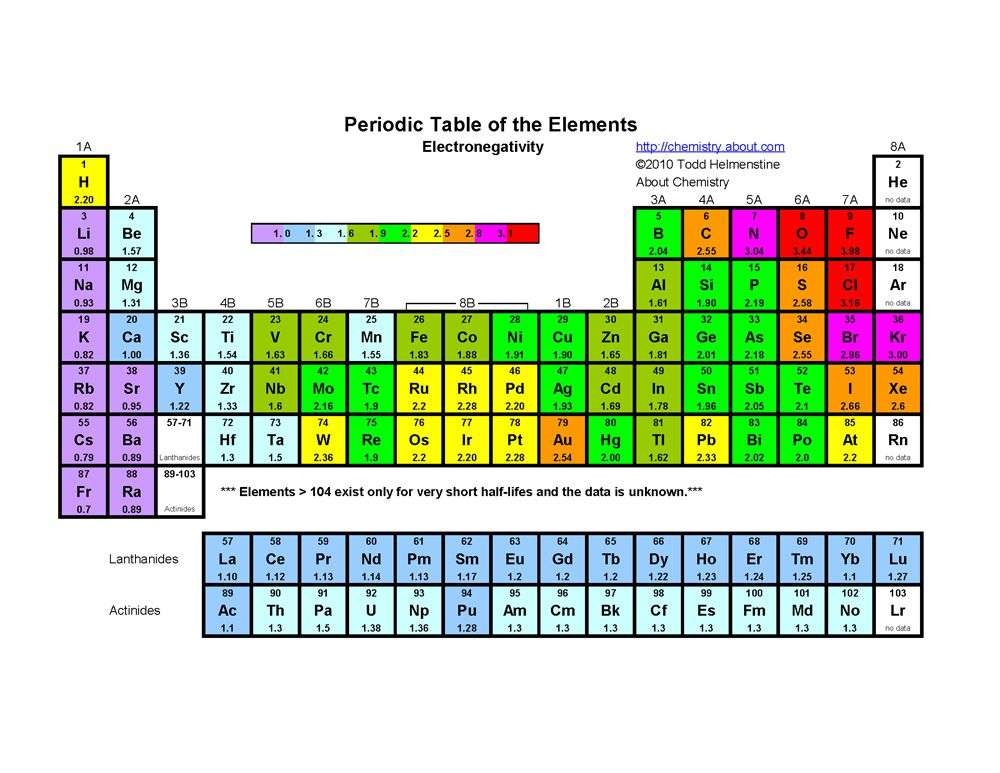
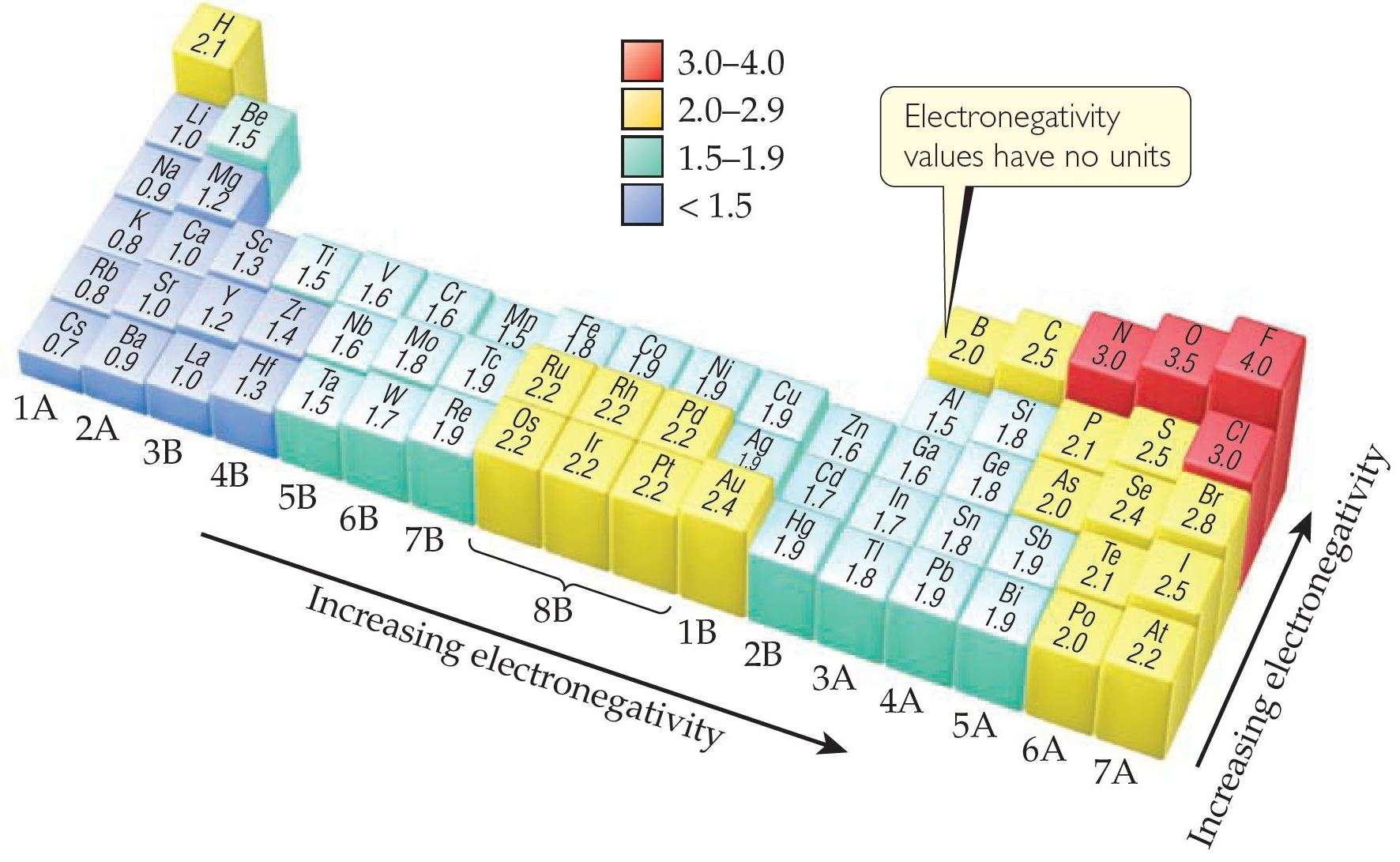
The electronegativity of an atom depends upon its atomic number and its atomic radius, which means that the more the distance between the nucleus and its valence electrons, the lower the electronegativity and vice versa. Electronegativity in the period table increases as you move from left to right across a period and as you move from top to bottom in a group.
Electronegativity not only helps us in studying the chemical properties of an atom but also plays a significant role in studying the electron affinity, type of bond formed between atoms, the magnitude of the bond’s polarity, and the bond order between bonding atoms.
On the periodic table, the electronegativity values of most of the elements have been calculated using the Pauling scale.
How To Determine Electronegativity
Electronegativity determination of surface atoms solely by experiments . Magnitude relationships of electronegativities for Si,Al, and unknown X atoms. Short-range E curves obtained on Si , Al adatoms, and SiO2 , respectively. The expected values of bond energy on SiO2 acquired by the Al tip are represented by light and dark green lines with their error bands. The inset shows a typical topographic AFM image of Si, Al adatoms, and SiO2 on the Si- surface. The ball-and-stick model of SiO2 represents Si in cream and O in red. Periodic table of the new thermo-chemical electronegativ values.
Don’t Miss: Elastic Force Equation
Most And Least Electronegative Elements
Fluorine is the most electronegative element on the periodic table. Its electronegativity value is 3.98. Cesium is the least electronegative element. Its electronegativity value is 0.79. Electro positivity is the exact opposite of electronegativity, therefore, we can say that Cesium is the most electropositive element.
Those elements requiring only a few electrons to complete their valence shells, and having the least quantity of inner electron shells between the positive nucleus and the valence electrons, are the most electronegative. The most electronegative of all elements are fluorine. Its electronegativity is 4.0. Metals have electronegativities less than 2.0. The least electronegative elements are cesium and francium , with electronegativity values of 0.7.
Therefore,
Fluorine is the most electronegative element and cesium is the least electronegative element.
What Is The Electronegativity Difference
The degree to which an atom attracts electrons in a chemical bond is described by electronegativity. If the difference in electronegativity is greater than 1.7, the character of the bond will be ionic. If the difference in electronegativity is between 0.4 and 1.7, the character of the bond is polar covalent.
Read Also: Holt Geometry Chapter 7 Test Answer Key
Patterns Of Electronegativity In Periodic Table
Fluorine is the most electronegative element in the periodic table. So, in groups and periods electronegativity always increases towards fluorine.
Image will be uploaded soon
Lets discuss its trend across a period and a group in detail
Electronegativity trend across a period As we move across a period electronegativity increases. The graph below shows electronegativities from sodium to chlorine which are increasing. We didnt include argon as it is an inert gas and dont form bonds with other elements.
Image will be uploaded soon
Electronegativity trend across a group As we move down across a group, electronegativity decreases. While if go up across a period electronegativity increases. The graphs below show electronegativity pattern across group 1 and 7
Image will be uploaded soon
This was brief on electronegativity, if you are looking for detailed notes on the topic explaining the factors affecting it etc. then register yourself on Vedantu or download Vedantu learning app for class 6-10, IIT JEE and NEET.
Trends In Electronegativity Across A Period
The positively charged protons in the nucleus attract the negatively charged electrons. As the number of protons in the nucleus increases, the electronegativity or attraction will increase. Therefore electronegativity increases from left to right in a row in the periodic table. This effect only holds true for a row in the periodic table because the attraction between charges falls off rapidly with distance. The chart shows electronegativities from sodium to chlorine .
You May Like: What Is Harder Chemistry Or Physics
Impact Of Electronegativity On Covalent Bonding
The strength of a covalent bond is highly dependent on the electronegativities of the two bonded atoms . Homonuclear diatomic molecules feature relatively pure covalent bonds since the electronegativities of the bonded atoms are the same . Examples of such covalent bonds can be seen in H2 molecules, Cl2 molecules, and O2 molecules.
On the other hand, the covalent bonds between two species of varying electronegativities tend to become polarized. This occurs because the more electronegative atom pulls the bond pair of electrons closer to itself, developing a partially negative charge in the process . At the same time, the more electropositive atom develops a partial positive charge . These partial charges are responsible for the polarity of the chemical bond.
Electronegativity Chart How To Find Electronegativity
If you want to calculate the electronegativity difference or the type of bond between two elements, you need to have an electronegativity chart for the electronegativity values of all elements on the periodic table.
Follow the given steps to calculate the electronegativity or chemical bond type:
Note the electronegativity of the first and second elements. How to find electronegativity? Just use a periodic table which includes it and read it for the selected element.
Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms.
Three different conditionsdetermine the type of chemical bond that the selected elements may form.
Now, compare the electronegativity difference you obtained with these three conditions to identify the bond.
For example, the electronegativity value of hydrogen is 2.20, and fluorine is 3.98. Their electronegativity difference is 1.78. The value lies between 0.4 and 2.00, implying that the bond type is polar covalent.
Recommended Reading: Chapter 10 Test Form 2b Answers
Patterns Of Electronegativity In The Periodic Table
The distance of the electrons from the nucleus remains relatively constant in a periodic table row, but not in a periodic table column. The force between two charges is given by Coulombs law.
In this expression, Q represents a charge, k represents a constant and r is the distance between the charges. When r = 2, then r2= 4. When r = 3, then r2 = 9. When r = 4, then r2 = 16. It is readily seen from these numbers that, as the distance between the charges increases, the force decreases very rapidly. This is called a quadratic change.
The result of this change is that electronegativity increases from bottom to top in a column in the periodic table even though there are more protons in the elements at the bottom of the column. Elements at the top of a column have greater electronegativities than elements at the bottom of a given column.
The overall trend for electronegativity in the periodic table is diagonal from the lower left corner to the upper right corner. Since the electronegativity of some of the important elements cannot be determined by these trends , we have to memorize the following order of electronegativity for some of these common elements.
F > O > Cl > N > Br > I > S > C > H > metals
The most electronegative element is fluorine. If you remember that fact, everything becomes easy, because electronegativity must always increase towards fluorine in the Periodic Table.
Why Does Electronegativity Increase Across A Period
Consider sodium at the beginning of period 3 and chlorine at the end . Think of sodium chloride as if it were covalently bonded.
Both sodium and chlorine have their bonding electrons in the 3-level. The electron pair is screened from both nuclei by the 1s, 2s and 2p electrons, but the chlorine nucleus has 6 more protons in it. It is no wonder the electron pair gets dragged so far towards the chlorine that ions are formed. Electronegativity increases across a period because the number of charges on the nucleus increases. That attracts the bonding pair of electrons more strongly.
Read Also: Jonathan Thomas Child Of Rage Now
Electronegativity In Covalent Bonds
In a covalent bond, the electron pair/s is shared between two atoms, making it stable. If the two atoms bonded have a similar electronegativity value,the bonded electron stays in the middle and both the atoms have an equal sharing. For example, if two Fluorine atoms are bonded, the bonded electron will stay in the middle.
However, if the electronegativity of the two atoms is not similar, the electrons that bonded will get pulled towards the atom with the higher electronegativity. This will lead to an unequal distribution of electrons and causes a dipole to be formed. A dipole points towards the more electronegative atom and shows where the electrons are being attracted to more and moving towards which atom.
As the electrons move towards the more electronegative atom, a time comes when the more electronegative atom attracts a large concentration of negative electrons. The concentration of electrons develops a partially negative charge over the more electronegative molecule. The less electronegative molecule has an electron depletion and thus develops a partial negative charge. The partial negative charge is represented with -. The partial positive charge is represented by +. The atom towards which the dipole points to becomes partially negative and the other atom becomes partially positive.
Bonds Between Highly Electronegative And Highly Electropositive Atoms
In the covalent bonds featuring a large difference in the electronegativities of the bonded atoms, it is not uncommon for the more electronegative atom to gain complete control over the bond pair of electrons, resulting in the formation of two ions. Here, the more electronegative atom forms an anion and the more electropositive atom becomes a cation.
It is important to understand that all covalent bonds between dissimilar species have some ionic character. Similarly, all ionic bonds have some covalent character as well. The ionic character of the covalent bond is determined by the difference in electronegativity. When the electronegativities of the bonded species are not very different, the bond will be more covalent than ionic. However, when there is a large enough difference in the electronegativities of the bonded atoms, the bond becomes polar enough to be considered more ionic than covalent.
Recommended Reading: Ccl4 Molecular Shape
Explaining The Diagonal Relationship With Regard To Electronegativity
Electronegativity increases across the Periodic Table. So, for example, the electronegativities of beryllium and boron are:
| Be | |
| B | 2.0 |
Electronegativity falls as you go down the Periodic Table. So, for example, the electronegativities of boron and aluminum are:
| B | |
| Al | 1.5 |
So, comparing Be and Al, you find the values are exactly the same. The increase from Group 2 to Group 3 is offset by the fall as you go down Group 3 from boron to aluminum. Something similar happens from lithium to magnesium , and from boron to silicon . In these cases, the electronegativities are not exactly the same, but are very close.
Similar electronegativities between the members of these diagonal pairs means that they are likely to form similar types of bonds, and that will affect their chemistry. You may well come across examples of this later on in your course.
The Electronegativity Of Chlorine Fluorine And Oxygen
Fluorine is the most electronegative element on the electronegativity chart, followed by oxygen and then chlorine. This has several implications. Firstly, it means that fluorine is always negative when combined with other elements. Secondly, it means that oxygen always has a negative oxidation state, except in the very rare case where it forms a compound with fluorine. This also explains the high reactivity of fluorine, chlorine and oxygen. Fluorine is so electronegative, that it wants to rip an electron off anything it touches.
Also Check: Does Mj Have Any Biological Kids
Periodic Trends In The Electronegativities Of Elements
As we move across a period from left to right the nuclear charge increases and the atomic size decreases, therefore the value of electronegativity increases across a period in the modern periodic table. For example, the electronegativity trend across period 3 in the periodic table is depicted below.
There is an increase in the atomic number as we move down the group in the modern periodic table. The nuclear charge also increases but the effect of the increase in nuclear charge is overcome by the addition of one shell. Hence, the value of electronegativity decreases as we move down the group. For example, in the halogen group as we move down the group from fluorine to astatine the electronegativity value decreases and it is shown in the diagram below.
It is a general observation that metals show a lower value of electronegativity as compared to the non-metals.Therefore, metals are electropositive and non-metals are electronegative in nature. The elements in period two differ in properties from their respective group elements due to the small size and higher value of electronegativity.
The elements in the second period show resemblance to the elements of the next group in period three. This happens due to a small difference in their electronegativities. This leads to the formation of a diagonal relationship.
Electronegativity And Group Electronegativity
12.14.1Electronegativity
Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons toward itself. The electronegativity of an atom is affected by both its atomic number and the distance that its valence electrons reside from the charged nuclei. The concept of electronegativity was first proposed by Pauli in 1932 as an explanation of the fact that the covalent bond between two different atoms is stronger than would be expected by taking the average of the strengths of the AA and BB bonds. This additional stabilization of the heteronuclear bond has been explained by the valence bond theory due to the contribution of the ionic canonical forms to the bonding. Electronegativity is a relative term and only the differences in the electronegativity are defined. Thus, Pauli proposed
Robert J. Ouellette, J. David Rawn, in, 2018
Don’t Miss: Calculating Half-life
Why Does Electronegativity Fall As You Go Down A Group
As you go down a group, electronegativity because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Consider the hydrogen fluoride and hydrogen chloride molecules:
The bonding pair is shielded from the fluorine’s nucleus only by the 1s2 electrons. In the chlorine case it is shielded by all the 1s22s22p6 electrons. In each case there is a net pull from the center of the fluorine or chlorine of +7. But fluorine has the bonding pair in the 2-level rather than the 3-level as it is in chlorine. If it is closer to the nucleus, the attraction is greater.
Which Element Is The Least Electronegative
Cesium is the least electronegative element on the periodic table. Its electronegativity value is 0.73.
Its atomic number is 55 u. It belongs to the 1st period and 6th group, alkali metal of the periodic table.
Cesium is one of the only five metal elements that are in the liquid state at room temperature.
Don’t Miss: Calculating Half Lives
Electronegativity And Oxidation State
Electronegativity values are helpful in determining oxidation numbers in cases where it might otherwise be difficult to decide which atom has the positive and which the negative oxidation number. In binary covalent compounds and polyatomic ions such as PO43- or ClO4, the more electronegative atom is arbitrarily assigned the negative oxidation number corresponding to the charge it would have if it were present as an ion. This leaves assignment of a positive oxidation number to the less electronegative element. As a result, oxidation numbers represent the relative electronegativities of atoms and the direction of the polarity of a bond. For example, in iodine trichloride, ICl3, chlorine is assigned the -1 oxidation number because it is the more electronegative of the two atoms. To achieve the necessary neutrality, iodine is assigned an oxidation number of + 3 in this compound.
where a, b, c are the numbers of atom of a given element .
As an application of the electronegativity equalization principle , Parr and Pearson derived Eqs. and to measure the amount of charge transfer N and the energy change E associated with the formation of A:B complex from acid A and base:B.
These expressions are very useful in understanding the acid-base reaction mechanism. It is important to note that the electronegativity difference drives the electron transfer whereas the hardness sum provides a resistance to it. Therefore both and are to be considered in analyzing these processes.
Electronegativity Increases Across The Period
Moving from left to right across a period, the number of protons and electrons increases while the number of energy shells stay same. Thus because of more attraction between increasing number of positive nucleus and negative electrons, the atomic radius decreases and the electronegativity increases across the period.
Don’t Miss: Is Physics Harder Than Math