Cis & Trans On Cyclic Compounds
Ring structures or cyclic compounds can also exhibit cis/trans isomerism without the presence of a pi bond.
Remember, substituents will be cis and trans if they are locked in place. Pi bonds are one way to lock them in place. Rings are another matter.
For example, in 1,2-dimethylcyclohexane, I can show both substituents going into the page or both going out of the page.
Since theyre pointing in the same direction, they are cis to each other.
If I show one going into the page and one going out of the page. They are trans to each other.
Even though the carbons are sp3 and sigma bound to each other, the molecule itself cannot rotate because of the ring structure. Locked.
The only way to turn cis-1,2-dimethylcyclohexane into trans-1,2-dimethylcyclohexane, is to break open the ring, rotate, and reform the ring.
Common Definitions And Terms In Organic Chemistry
© Dr Jeffrey Gosper and Prof. Peter Sammes, Brunel University, 1995.
These definitions are the preferred ones to be used in Organic Chemistry. Notethat some terms have more than one interpretation.
acid: an agent able to produce positively charged hydrogen ions.
achiral: not chiral. A compound that is superimposible on its mirror image.For example CH4.
activation energy: the miminmum energy which reacting species mustpossess in order to be able to form an ‘activated complex’, or ‘transitionstate’, before proceeding to the products.
addition reactions: reactions in which an unsaturated system issaturated or part saturated by the addition of a molecule across the multiplebond.
alkaloid: organic substances occurring naturally, which are basic,forming salts with acids. The basic group is usually an amino function.
allyl group: a group containing 3 carbon atoms and a double bond.
allylic rearrangement: the migration of a double bond in a 3-carbonsystem from carbon atoms one and two to carbon atoms two and three,
e.g. C1=C2-C3-X X-C1-C2=C3
anomers: the specific term used to describe carbohydrate stereoisomersdiffering only in configuration at the hemi-acetal carbon atom.
association: a term applied to the combination of molecules of asubstance with one another to form more complex systems.
asymmetry: a term applied an object or molecule that does not possesssymmetry.
Avogadro’s constant: the number of particles in onemole of any pure substance.
AB = A + B
Physical Differences Between Cis And Trans Molecules
There are many differences in the physical properties of cis- and trans- isomers. Cis- isomers tend to have higher boiling points than their trans- counterparts. Trans- isomers generally have lower melting points and have lower densities than their cis- counterparts. Cis- isomers collect the charge on one side of the molecule, giving the molecule an overall polar effect. Trans- isomers balance the individual dipoles and have a non-polar tendency.
Read Also: How To Avoid Parallax Error In Physics
What Are Z Isomers
Z isomers are alkenes having the substituents with higher priority on the same side of the double bond. The letter Z comes from zusammenin German, which means together.
Figure 02: E-Z nomenclature of 2-butene
In the above image, the substituents with high priority are on the same side of the double bond in the Z isomer whereas the E isomer has those substituents in the opposite sides. Furthermore, the CIP rules determine the priority of these substituents. For the above example, the atoms directly bonded to the double bonded carbon are Carbon atoms of methyl groups and hydrogen atoms . Since carbon atom has a high atomic number when compared with hydrogen , the high priority is to the methyl group .
When Do We Use Cis
We use the terms cis- and trans to denote the relative configuration of two groups to each other in situations where there is restricted rotation.
In nomenclature, cis is used to distinguish the isomer where two identical groups are pointing in the same direction from the plane of the ring, and trans to distinguish the isomer where they point in opposite directions.
A common name for these so-called cis-trans isomers is geometric isomers.
In order for cis- trans- isomerism to exist in rings, we need two conditions:
- two carbons each bearing non-identical substituents above and below the ring
- the two carbons have at least one of those substituents in common
In 1,2-dichlorocyclopentane we saw that C-1 and C-2 each had non-identical substituents above and below the ring, and they each had at least one substituent in common .
Heres another example: cis- and trans 1-ethyl-2-methylcyclobutane. Note that they each have two carbons which each bear non-identical substituents above and below the ring . They also have at least one substituent in common . So we can refer to cis-1-ethyl-2-methylcyclohexane as the isomer where the two hydrogens are pointing in the same direction, and trans where they point in opposite directions.
If youve covered chirality, you might also note an interesting fact: there are two ways to draw each of the cis- and trans isomers, and they cant be superimposed on each other. These are enantiomers, by the way.
Don’t Miss: Geometry Dash Theory Of Everything 2
E Is For Eeposite Z Is For Ze Zame Zide
If the two high priority groups are opposite to each other, think of them as being eeposite to each other.
E is for Eeposite.
If the two high priority groups are on the the same side, or should I say on Ze Zame Zide,’ they are Z.
This applies to molecules that have more than just 1 carbon on either side of double bond.
Ze Zame Zide.
Lets go back to the example above:
On the left, OH outranks ethyl since oxygen has a higher atomic number when compared to carbon. OH is #1 and points down.
On the right, Cl outranks methyl since chlorine has a higher atomic number when compared to carbon. Cl is #1 and points down.
Since both arrows point in the same direction , we conclude that the priority groups are on Ze Zame Zide making it Z.
What Does Iso Mean For Organic Chemistry
3.9/5isomeansorganicmeansorganicanswer here
The prefix iso-, which stands for isomer, is commonly given to 2-methyl alkanes. In other words, if there is methyl group located on the second carbon of a carbon chain, we can use the prefix iso-. The prefix will be placed in front of the alkane name that indicates the total number of carbons.
what is an isopropyl group? Illustrated Glossary of Organic Chemistry – Isopropyl group. Isopropyl : A portion of a molecular structure equivalent to propane minus one hydrogen atom from the middle carbon. Sometimes abbreviated as iPr.
Accordingly, how do you use ISO in organic chemistry?
The prefix “iso” is used when all carbons except one form a continuous chain. This one carbon is part of an isopropyl group at the end of the chain. “Iso” can also indicate that the molecule is an constitutional isomer of another molecule with a common name.
What are the prefixes in organic chemistry?
Organic Chemistry Prefixes
Recommended Reading: Prince Jackson Biological Father
Breaking Ties: The Method Of Dots
For instance, the alkene below presents us with a dilemma: one of the carbons of the alkene is attached to two carbon atoms. So how do we determine priorities in this case. How do we break ties?
In the case of ties, we must apply the method of dots. Dots are handy placeholders which is why I like to use this method.
- Place a dot on each of the two atoms you are comparing.
- List the 3 atoms each atom is attached to, in order of atomic number.
- Compare the lists, much like you would compare a set of three playing cards. Just as a hand of would beat , so would beat .
- If the lists are identical, move the dots outward to the highest priority atom on the list.
- At the first point of difference, assign .
- If there is no difference then the groups are identical, and E / Z does not apply.
Heres a practical example of the method of dots.
Heres a more complex example with multiple alkenes. In this case each pi bond is designated by a number with its own separate E or Z configuration.
OK, this was long. But hopefully useful.
Watch out for a future post in which we go into more detail on the method of dots.
Other Types Of Isomerism
Stereoisomers may be described using other notation besides cis- and trans-. For example, E/Z isomers are configurational isomers with any rotational restriction. The E-Z system is used instead of cis-trans for compounds that have more than two substituents. When used in a name, E and Z are written in italic type.
Read Also: Who Are The Biological Parents Of Prince Paris And Blanket
Difference Between E And Z Isomers
April 23, 2018 Posted by Madhu
The key difference between E and Z isomers is that E isomers have the substituents with higher priority in the opposite sides whereas the Z isomers have the substituents with higher priority on the same side.
The E-Z nomenclature is a notation system to name different isomers having the same chemical formula, but different spatial arrangements. Furthermore, the E and Z isomers are alkenes. These isomers get their name based on the position of the substituents attached to the double bond of the alkene.
What Are E Isomers
The E isomers are alkenes having the substituents with higher priority on the opposite sides of the double bond. The letter E comes from entgegen in German, which means opposite. The basis of E-Z notation is a set of rules known as priority rules. They are the Cahn-Ingold-Prelog rules. These are a set of rules to name the organic molecules, to specify them unequally.
The steps of naming a molecule using CIP rules are as follows
Also Check: Who Are Paris Jackson’s Biological Parents
Do You Have To Have Chiral Carbons To Have Enantiomers And Diastereomers
No, you do not! Note how the definition of enantiomers says that the molecules are non-superimposable mirror images, while diastereomers are non-superimposable non-mirror images? The definitions say nothing about the chiral centers or atoms. Thus, any pair of molecules that fits the definition, works! For instance, allenes are cumulated alkenes that are not planar:
If you build a pair of allenes with your molecular model kit , youll see, that those are not superimposable in space. But these two molecules have no chiral carbons and yet, they fit the definition of enantiomers, therefore they are a pair of enantiomers! So, as you can see, the chiral atoms by themselves is not the important thing here, rather its the 3D structure of the molecule itself.
You can also have diastereomers in molecules that dont have any chiral atoms. Look at the following examples:
The top pair is an example of the cis/trans isomers in alkenes. The second pair also represents as cis/trans pair of isomers. Neither of molecules, however, have chiral atoms. And since each pair represents a couple of non-superimposable molecules that are not mirror images, they are diastereomers.
Cis Trans And E Z Geometric Isomers

Your organic chemistry course will cover many different types of isomers.
Isomers have the same molecular formula but something about them is different.Geometric isomers, a type of stereoisomer, differ in their geometry or shape. This happens when substituents are LOCKED in a specific relationship to each other.
I say locked because, unlike conformational isomers in Newman Projections, you cant simply rotate the molecule to change the relationship between substituents.
In this tutorial, well look at alkene geometric isomers including cis trans and E Z.
Recommended Reading: Is Paris Jackson Michael’s Biological Daughter
What If Theres More Than One Substituent On The Sp2 Carbon
Till now, weve looked at molecules with just one substituent on either side of the sp2 pi bound carbon.
What happens if we have a pi bond with 2 different atoms or groups on the sp2 carbon?
Take a look at 3-methyl-2-pentene:
Here in line structure:
You can draw this molecule in 2 different ways. But will you compare the red methyl or red ethyl to the green methyl when choosing cis or trans?
While some professors WILL teach you to compare the larger groups, the answer is that you CANNOT compare simply choose one for cis and trans.
Naming E And Z Alkenes
The E and Z designation for the configuration of a double bond is also included in the nomenclature of alkenes.
As an example, lets name the alkene for which we have just determined the E/Z configuration above:
First, determine the name according to the IUPAC rules. The parent chain is heptane and there are three substituents:
Additionally, the configuration of the alkene is placed before the systematic name:
Below are some practice problems for determining the E and Z configuration of alkenes:
Recommended Reading: Geometry Segment Addition Postulate Worksheet
The Priorities Of The Substituents
- First consider the atoms directly bonded to the chiral center or the double bond higher the atomic number, higher the priority
- If there are equal atoms present, then there is a tie. Then check the substituent groups to find a point of difference in atomic number.
- If still there is a tie, consider the atoms bonded to each of the atoms in the main chain and check whether there is a point of difference.
Figure 01: E-Z nomenclature of 3-methylpent-2-ene
In the above image, the E isomer has high priority substituents on the opposite sides of the double bond whereas the Z isomer has those substituents on the same side.
When determining the priority of the substituents, first consider the atoms directly bonded to the double bond in the above example, there are three Carbon atoms and one hydrogen atom . Therefore, there is a tie because one of the two vinyl carbon atoms have directly bonded carbon atoms. Then, to determine the high priority group, consider the atom that comes after these directly bonded carbon atoms. Since the substituent group attached to this vinyl carbon are a methyl group and an ethyl group , the priority is given to the ethyl group. That is because the atom comes after the directly bonded carbon atom is a hydrogen atom in methyl group and a carbon in the ethyl group.
Heres My Simple Approach
You May Like: Geometry Segment Addition Postulate Worksheet
Understanding Peaks Like Singlet Doublet Triplet Quartet And Multiplet
ByLeah Fisch | Submitted On January 30, 2013
As an organic chemistry student you will likely come across the topic of hydrogen spectroscopy more specifically referred to as H-NMR hydrogen or proton NMR.There are many aspects that you will have to analyze on the graph, the most important of which are the types of splitting exhibited by the individual peaks. In this article I will explain the name and appearance of the basic signals
Splitting on H-NMR comes from hydrogen neighbors. If you isolate the hydrogen atoms in question, you want to look at the carbon holding these H’s, and then see how many hydrogen atoms are attached DIRECTLY to the neighboring carbon. This allows you to form the following connectivity
H – C – C – H
A hydrogen atom can have anywhere from zero 9 hydrogen neighbors
Every hydrogen starts out a single peak when analyzed by itself. When analyzed in the presence of it’s neighbors, every peak will be split once, meaning you add an additional tip, for each extra hydrogen neighbor
SingletA singlet, as the name implies is a single peak without any splitting. This implies that the hydrogen responsible for its peak does not have any H-neighbors
DoubletWhen you see 2 tall tips on your graph you have what is considered a doublet. This implies that the hydrogen responsible for this peak has just 1 H-neighbor. A doublet will have 2 tips that are approximately the same height
Quintet: 4 neighbors
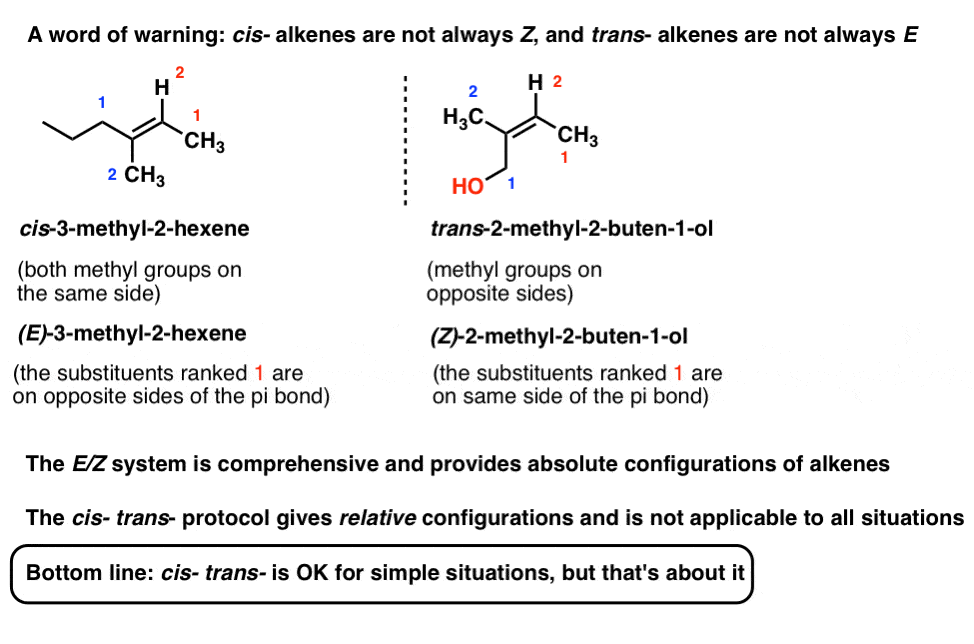
Ez Will Always Work Even When Cistrans Fails
In simple cases, such as 2-butene, Z corresponds to cis and E to trans. However, that is not a rule. This section and the following one illustrate some idiosyncrasies that happen when you try to compare the two systems. The real advantage of the E-Z system is that it will always work. In contrast, the cis-trans system breaks down with many ambiguous cases.
Example 2
The following figure shows two isomers of an alkene with four different groups on the double bond, 1-bromo-2-chloro-2-fluoro-1-iodoethene.
It should be apparent that the two structures shown are distinct chemicals. However, it is impossible to name them as cis or trans. On the other hand, the E-Z system works fine… Consider the left hand structure. On C1 , the two atoms attached to the double bond are Br and I. By the CIP priority rules, I is higher priority than Br . Now look at C2. The atoms are Cl and F, with Cl being higher priority. We see that the higher priority group is “down” at C1 and “down” at C2. Since the two priority groups are both on the same side of the double bond , they are zusammen = together. Therefore, this is the isomer. Similarly, the right hand structure is .
You May Like: Laws Of Exponents Worksheets 8th Grade
The First Point Of Difference Rule
Which is higher priority, by the CIP rules: a C with an O and 2 H attached to it or a C with three C? The first C has one atom of high priority but also two atoms of low priority. How do these “balance out”? Answering this requires a clear understanding of how the ranking is done. The simple answer is that the first point of difference is what matters the O wins.
To illustrate this, consider the molecule at the left. Is the double bond here E or Z? At the left end of the double bond, Br > H. But the right end of the double bond requires a careful analysis.
At the right hand end, the first atom attached to the double bond is a C at each position. A tie, so we look at what is attached to this first C. For the upper C, it is CCC . For the lower C, it is OHH — listed in order from high priority atom to low. OHH is higher priority than CCC, because of the first atom in the list. That is, the O of the lower group beats the C of the upper group. In other words, the O is the highest priority atom of any in this comparison thus the O “wins”.
Therefore, the high priority groups are “up” on the left end and “down” on the right end . This means that the isomer shown is opposite = entgegen = E. And what is the name? The “name” feature of ChemSketch says it is -2-pent-3-ynyl methyl ether.