Hypotonic Solution Definition In Biology
In biology, one can define a hypotonic solution as a solution that has lower osmotic pressure than the other solution it is compared with. In other words, it is a solution that has a lower amount of solute as compared to the solute concentration in the other solution across a semipermeable membrane. A cell exposed to a hypotonic solution will eventually swell as the water molecules enter the cell via passive transport.
Hypotonicity is therefore a relative term whereby the property of the solution is defined relative to the other solution. Most times, in biology the solution of comparison is the cytosolic fluid or the fluid present inside a cell. This means a solution would be described as hypotonic when it has a lower amount of solutes than the cytosol of a cell and the semipermeable membrane is usually the cell membrane.
In biology, scientists use hypotonicity to enable them to describe cells. A scientist knowing the osmolarity of different solutions can enable the scientist to know which way the solute gradients and water gradients will form. Osmolarity is defined as the concentration of a solution in number of solutes per liter. Furthermore, hypotonic solutions are classified in biology, with reference to blood serum. Therefore, with respect to blood serum, the solutions that have osmolarity that is less than 280mOsm/liter are referred to as hypotonic solutions.
What Happens To A Cell As It Is Placed In A Hypertonic Solution
molecules when it is placed in hypertonic solution*.
Explanation:
Hypertonic solution is the one which contain more concentration of solutes as compared to the concentration of solutes in cytoplasm of cell. Which means that a solution contains less water as compared to the water within the cell.
So, When cell is placed in this kind of solution then water ## molecules move from their higher concentration to their lower concentration i.e from cell to hypertonic solution. This movement of water molecule is called osmosis and it is due to the fact that everything in the universe tends to attain equilibrium. Water molecules also try to balance their concentration in both environments. Thus, cell gives its water to the solution in which it has been placed. Hence, it loses water and gets shrunk.
The image below manifests: what happens to red blood cells when it’s placed in hypertonic solution?
Hope it helps!
Refer to the explanation.
Explanation:
We usually talk about water molecules in cells placed in a hypertonic solution. A hypertonic solution has a higher concentration of dissolved solutes, and a lower concentration of water compared to the cell. Since the cell membrane is semi-permeable, not all solutes can cross the cell membrane, but water can always cross the cell membrane.
So, water will move from the cell to the hypertonic solution from where it is in higher concentration to where it is in lower concentration . This process is called osmosis.
What Are The Examples Of Hypertonic
Hypertonic Solution Examples
- Seawater. Seawater has a high amount of salt particles compared to freshwater, making it a hypertonic solution.
- Sugary Drinks. Have you ever tasted a sugary drink that was so sweet it made your mouth pucker?
- Extracellular Fluid in Hypertonic Dehydration.
- IV Drips and Injections.
Recommended Reading: How To Find Precision In Chemistry
What Is A Hypertonic Solution
Science Photo Library-STEVE GSCHMEISSNER./Getty Images
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Hypertonic refers to a solution with higher osmotic pressure than another solution. In other words, a hypertonic solution is one in which there is a greater concentration or number of solute particles outside a membrane than there are inside it.
What Are The Isotonic & Hypertonic Solutions
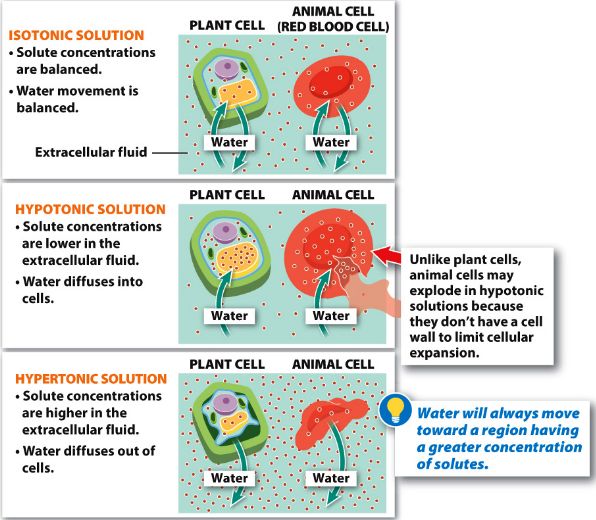
Isotonic solutions are most commonly used for regular maintenance of clean, healthy sinuses â a soothing wash to remove excess mucus, dust, and allergens â and for helping address such things as post-nasal drip. A hypertonic solution contains a higher concentration of salt than your bodyâs fluids.
Also Check: What Does I Mean In Chemistry
What Is Hypotonic Solution
Hypo denotes low, hence a hypotonic solution is one that has a higher water content than solute concentration. A solution is a molecularly dispersed mixture of one or more compounds in a sufficient quantity of dissolving solvent. A solute is a dissolved material in a solution. What enters and leaves the cell is regulated and controlled by the cell membrane. A selectively permeable membrane is a membrane that enables certain materials to pass through but not others.
In a hypotonic solution, the solute concretion is always smaller than the cell. There is less solvent because there is a high concentration of solute inside the cells .
Osmosis is a process in which water passes through a semipermeable membrane from a high concentration to a low concentration. The fact that the solute concentration is low in this solution implies that the solvent concentration is high. Water flows from the outside to the inside of the cell.
Where Does Water Move In A Hypertonic Solution
If a cell is placed in a hypotonic solution, water will move into the cell. This causes the cell to swell, and it may even burst. A hypertonic solution means the environment outside of the cell has more dissolved material than inside of the cell. If a cell is placed in a hypertonic solution, water will leave the cell.
Born and raised in the city of London, Alexander Johnson studied biology and chemistry in college and went on to earn a PhD in biochemistry. After completing his doctoral studies, he decided to start “ScienceOxygen” as a way to share his passion for science with others and to provide an accessible and engaging resource for those interested in learning about the latest scientific discoveries. In his writing, Alexander covers a wide range of topics, from cutting-edge medical research and technology to environmental science and space exploration. He also shares personal stories and insights from his own journey as a scientist and researcher.
Don’t Miss: Molecular Geometry Worksheet Answer Key
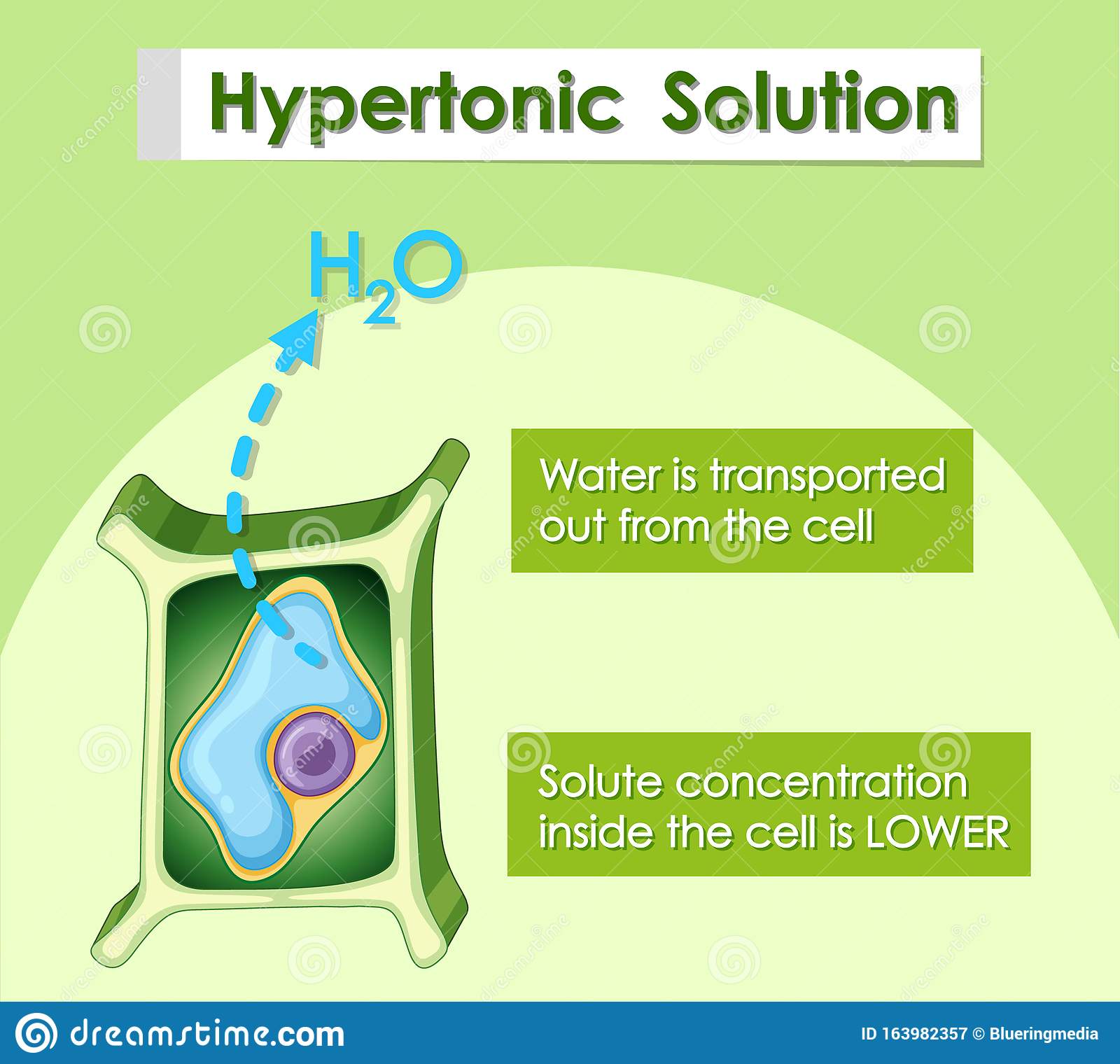
Hypertonic And Hypotonic Solution
A hypertonic solution is one in which the concentration of solutes in the solution is higher than the concentration of the cell in the solution. Saltwater is an example of a hypertonic solution. Whereas a hypotonic solution is a solution that has more water outside the cell. Water then travels into the cell from the solution.
Both animal and plant cells have been shown to be affected by hypertonic and hypotonic solutions. Hypertonic solution has a higher solute content and less water than a cell. There is a net migration of water from inside to outside the cell because the concentration of water is higher within the cell. Osmosis allows water to leave the cell. The loss of water causes the cells in a hypertonic solution to shrink. The animal cell will shrivel up in a hypertonic solution. This is known as crenation. The plant cell becomes less stiff in a hypertonic solution, which is known as plasmolysis.
In a hypotonic solution, The water enters the cell due to high solute concentration inside the cell. The water is more in this hypotonic solution and solute concentrations are low.
Image: Hypotonic solution
What Is Isotonic And Isometric
Isotonic muscle contraction produces limb movement without a change in muscle tension, whereas isometric muscle contraction produces muscle tension without a change in limb movement. Most physical activities involve a combination of both forms of muscle contraction, although one form usually predominates.
You May Like: What Are Probes In Biology
A Cell In Hypertonic Solution
The plasma membrane that surrounds cells is a special permeable membrane that separates the contents of the cell from the environment. The plasma membrane is embedded with special membrane transport proteins that help transport solutes across. It also has special protein channels called aquaporins that allow water to flow freely across the membrane. The cell must use energy to actively move solutes into and out of the cell. Too many solutes and the cytosol will become a hypertonic solution compared to the environment. Cells without cell walls can burst in this condition.
Too few solutes in the environment will become the hypertonic solution. In this case, the opposite will happen, as water moves out of the cell. Water moves against the concentration gradient of solutes, moving from areas of low solute concentration to areas of high solute concentration. In another sense, water moves with the water concentration gradient, from areas of high water concentration to areas of low water concentration.
Using Visuals To Help Explain Tonicity To Introductory Biology Students
BRIAN RAFFERTY is an Assistant Professor in the Science Department, Borough of Manhattan Community College, New York, NY 10007.
LALITHA JAYANT is a Professor in the Science Department, Borough of Manhattan Community College, New York, NY 10007.
Brian Rafferty, Lalitha Jayant Using Visuals to Help Explain Tonicity to Introductory Biology Students. The American Biology Teacher 1 March 2021 83 : 185187. doi:
The inability of students to properly understand the principles underlying osmosis and tonicity leads to misconceptions that further impair their ability to apply these concepts to physiological situations. We describe a simple and inexpensive visual exercise using beads and water to mimic solutions. Using these model solutions, students will understand the concepts of tonicity and osmolarity. The hands-on exercise is supplemented with a worksheet that reinforces the concepts they learned in doing the activity. This exercise has broad application with respect to both the level of students targeted and the courses in which it can be utilized, and it is flexible enough to personalize for each situation.
Recommended Reading: Can An Adopted Child Inherit From Biological Parents
Elizabeth Martin And Robert Hine
- Publisher:
- or copy the link directly:https://www.oxfordreference.com/abstract/10.1093/acref/9780199204625.001.0001/acref-9780199204625-e-2190 jsessionid=04645CA23BACB4165AA6DDA714C8C3C5The link was not copied. Your current browser may not support copying via this button.Link copied successfully
PRINTED FROM OXFORD REFERENCE . Copyright Oxford University Press, 2023. All Rights Reserved. Under the terms of the licence agreement, an individual user may print out a PDF of a single entry from a reference work in OR for personal use .
date: 23 January 2023
This Problem Has Been Solved
You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.
A hypertonic solution has a higher level of solutes so the watermoves out of the cells to the surroundings
A hypertonic solution has a higher level of solutes so the watermoves out of the cells to the surroundings
A hypotonic solution has a higher level of solutes so thewater moves out of the cells to the surroundings
The salt and water solution in the potato is a hypotonicsolution
Osmosis is the movement of large molecules across a membranewhich require embedded proteins
The plain water in this experiment is the solvent and the saltis the solute.
Passive transport requires no energy and the molecules move withthe concentration gradient or from high concentration to lowconcentration
Active transport required no energy and the molecules move withthe concentration gradient or from high concentration to lowconcentration.
The water on the inside of the potato with the salt molecules isan isotonic solution the same as the solution on the outside of thepotato.
Water will not move by simple diffusion into orout of cells it requires energy or protein transporters
The solution of salt water in the potato has a lowerconcentration of water molecules so the water on the outside shouldmove into the cells
Don’t Miss: Predict The Molecular Geometry And Polarity Of The So2 Molecule
The Plasma Membrane And Cytosol
If the outside environment of a cell is water-based, and the inside of the cell is also mostly water, something has to make sure the cell stays intact in this environment. What would happen if a cell dissolved in water, like sugar does? Obviously, the cell could not survive in such an environment. So something must protect the cell and allow it to survive in its water-based environment. All cells have a barrier around them that separates them from the environment and from other cells. This barrier is called the plasma membrane, or cell membrane.
Examples Of Hypertonic Solution
- When compared to freshwater, seawater contains a higher concentration of salt particles, making it a hypertonic solution. Freshwater fish cannot survive in seawater because their cells would leak water into the surrounding saltwater. They’d quickly perish from dehydration.
- Have you ever had a sugary drink that made your tongue pucker because it was so sweet? Because the drink included more sugar than water, it was a hypertonic solution. Because the water from your mouth surged into the drink,dehydrating your mouth, your lips puckered. Sugary drinks can also deplete the water in your intestinal cells, making it difficult to absorb nutrients. As a result, sports drinks have less sugar than other beverages.
- The amount of water in healthy blood cells is the same as the fluid around them. Your extracellular fluid becomes hypertonic and you become dehydrated if you sweat a lot or lose more water than sodium in other ways. Osmosis happens when fluid interacts with redblood cells, depleting them and preventing them from delivering oxygen.
You May Like: Are Subjects Of Psychological Research
What Is Difference Between Isotonic And Hypertonic
If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. In an isotonic environment, there is no net water movement, so there is no change in the size of the cell. When a cell is placed in a hypotonic environment, water will enter the cell, and the cell will swell.
Whats The Difference Between Hemolysis And Crenation
Summary Hemolysis vs Crenation Hemolysis occurs when red blood cells are in a hypotonic solution, causing red blood cells to swell up and burst due to water into the cells. Crenation occurs when red blood cells are in a hypertonic solution, causing red blood cells to shrivel due to water moving out of the cells.
Recommended Reading: What Does Composition Mean In Math
Uses Of Hypertonic Solutions
Manipulating the tonicity of a solution has practical applications. For example, reverse osmosis may be used to purify solutions and desalinate seawater.
Hypertonic solutions help to preserve food. For example, packing food in salt or pickling it in a hypertonic solution of sugar or salt creates a hypertonic environment that either kills microbes or at least limits their ability to reproduce.
Hypertonic solutions also dehydrate food and other substances, as water leaves cells or passes through a membrane to try to establish equilibrium.
What Type Of Solution Is Hypertonic
Hypertonic Solutions. Hypertonic solutions have a higher concentration of dissolved particles than blood. An example of hypertonic IV solution is 3% Normal Saline . When infused, hypertonic fluids cause an increased concentration of dissolved solutes in the intravascular space compared to the cells.
Don’t Miss: How Many Domains Are There In Biology
Understanding Cell Pressure Gradients
In animals, cells are always striving to maintain an equilibrium between their internal environment and the surrounding environment. The barrier between the cell and the outside world is a semipermeable membrane called the cell membrane. Besides water, the extracellular environment for cells in the human body includes plasma, proteins, fats, glucose, waste products, ions, and other substances. These dissolved materials are called solutes. Similar solutes are also present inside cells.
Osmosis is a spontaneous homeostatic process where water moves from an area of low solute concentration to high solute concentration through a semipermeable membrane. This is a natural process reflecting the preference of systems to achieve and maintain equilibrium. The amount of water outside a cell compared to the inside creates an osmotic pressure gradient which causes water to move. In other words, if there are more solutes outside the cell than inside, water will move out of the cell to equalize the solute level inside. Conversely, more solutes inside the cell compared to the outside environment causes water to enter the cell. The process by which organisms maintain water balance is called osmoregulation.
Cells In Aqueous Solutions
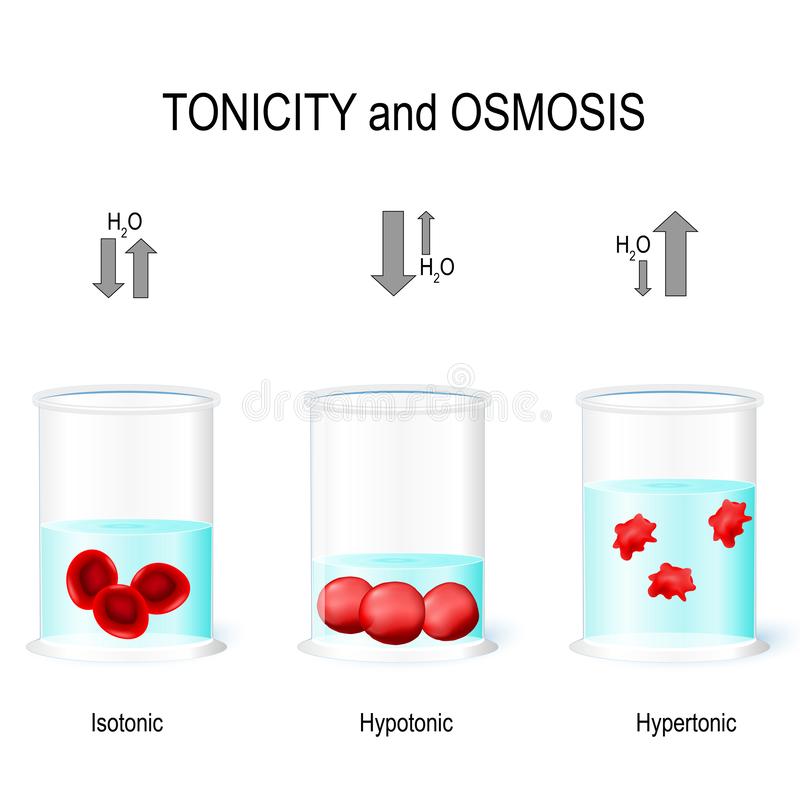
The figures show what can happen when animal or plant cells are placed in an aqueous solution. Water can move across membranes, but polar solutes dissolved in water cannot. The net movement of water is in the direction of increased solute concentrations. An easy way to visualize this rule is simply that the net water movement is from an area of high water concentration to an area of low water concentration .
Animal cells
Also Check: What Does Fitness Mean In Biology
Key Differences Between Hypertonic And Hypotonic Solution
Both the hypertonic and hypotonic solutions are based on the concept of tonicity and osmosis. By knowing the concept of tonicity and osmosis, we can understand the general idea about the direction and extension of the water movement in the solution.
Administration Of Hypotonic Solution
Hypotonic solutions are frequently employed to dilute extracellular fluid and rehydrate cells in individuals with hypertonic fluid imbalances, as well as to treat gastric fluid loss and dehydration caused by severe diuresis. This type of solution contains no calories or other electrolytes, but it does contain free water, salt, and chloride. The cell has a low amount of extracellular solute and wants to shift into the cell to utilise standard osmosis. This promotes cell swelling, which can result in the cell bursting or lysing.
0.45 percent saline , 0.225 percent saline , and 0.33 percent saline are hypotonic solutions. When a cell is dehydrated and fluids must be reintroduced, hypotonic solutions are used. This happens when a patient develops diabetic ketoacidosis or hyperosmolar hyperglycemia.
When we talk about Intravenous fluids, we usually mean that the water wants to exit the intravascular region and enter the Red Blood Cells . The most common cause of giving 0.45 percent salinity is real dehydration, which occurs when the body loses solely water and no electrolytes . The body already has a normal amount of electrolytes in dehydration. Therefore, there is no need to add extra to the IV solution. Only some of the patient’s water needs to be refilled.
Don’t Miss: What Is Secure Attachment In Psychology