Theoretical Derivation For Exploring The Effect Of Metalligand Interactions On Ligand Binding Affinities
| ) using eqn . The relationship between Gn and applies to any metalcoordinating atom interaction without displacement of the original coordinating atoms. It also applies to the metalcoordinating atom interaction that displaces a metal-bound water. However, the metalcoordinating atom interaction must be stronger than the metalwater interaction otherwise, all binding free energies are unfavorable. |
Because the bonding power trend for metal complexes shown in Fig. 5 is typical, the shielding effect shown in Fig. 5 is also typical. For a metalligand interaction, the newly formed metalligand interaction decreases the positive charge density of the metal ion, which then weakens the original interactions of the metal ion. The interactions of the coordinating atoms with larger negatively charged densities are weakened more and have a larger shielding effect. Therefore, stronger original interactions have a larger shielding effect than the weaker original interactions.
Shielding Effect And Ionization Energy
The larger the number of electrons in the inner shell, the lesser the attractive force holding the valence electron to the nucleus, and the lower will be the value of ionization energy. When we move down in the group of the periodic table, the number of shielding electrons increases, and the effective nuclear charge for valence electron calculate from Slaters rule decreases, and hence the ionization trends also decrease. Therefore, the ionization trends and shielding or screening effect for the group-2 chemical elements, beryllium> magnesium> calcium> strontium > barium. Hence the formation of ionic bonding or polarity trends also increases from Be to Ba.
Matt Hancock Vs The Sunday Times
This confusion was made apparent on 3 May, by the response that the health secretary, Matt Hancock, gave to the front page story in The Sunday Times.
The story said, correctly, that all those aged 70 and over, regardless of health conditions, have been classified as clinically vulnerable.
But it then incorrectly said that the clinically vulnerable have been asked to stay inside for at least 12 weeks, when this is actually the shielding advice given to the clinically extremely vulnerable. The piece has since been corrected.
Responding on , Mr Hancock described the story as factually wrong and misleading but made a mistake in his attempt to correct the article.
He said correctly that over 70s have not been asked to stay in lockdown for 12 weeks, but then incorrectly said that over 70s are not clinically vulnerable which they are.
The government advice on social distancing at the time, said people aged 70 or older are classed as clinically vulnerable, regardless of any medical conditions. It said these people are at higher risk of severe illness and should take particular care to minimise contact with others outside your household.
This is still the advice as of 4 June.
The NHS also lists people aged 70 or over as being clinically vulnerable, recommending they only leave home if it is essential.
But what do these two labels mean in practice?
Recommended Reading: Paris Jackson Dad
Experimental Validation For The Shielding Effect Of Metalligand Interactions On The Ligand Binding Affinity
| Fig. 6 The electrostatic interactions of ligands with sufficiently coordinated metal ions provide minimal little contribution to the ligand binding affinities. Structures and binding affinities of the six structurally similar inhibitors binding to carbonic anhydrase II47 . The interactions of a 5-coordinated zinc complex the interactions of a 4-coordinated zinc complex . The 5-coordinated zinc complex has one more ZnO interaction than the 4-coordinated zinc complex. However, the ZnO interaction has little contribution to ligand binding affinity. |
123+12121212123+3+12
| Fig. 7 Favorable binding free energy contributed by the interactions of hypo-coordinated metal ions. The structures of two inhibitors 1 and 2 and their binding affinities to protocatechuate 3,4-dioxygenase49 . Comparison of the interactions of the carboxyl groups in 1 and 2. Both carboxyl groups have strong interactions with Tyr324. The carboxyl group of 1 interacts with a restrained water molecule, whereas the carboxyl group of 2 interacts with bulk water. Comparison of the interactions of the metal ions (Fe3+ |
= 5.1 kcal mol1) approximately equals the binding free energy of the 5-coordinated Fe ion with a water molecule from bulk water. Because the OCN for Fe3+ is 6 and because the 5-coordinated Fe ion is hypo-coordinated , the binding of a hypo-coordinated ion with a coordinating atom may be largely favourable in terms of Gibbs free energy.
4
How Does The Shielding Effect Affect Trends
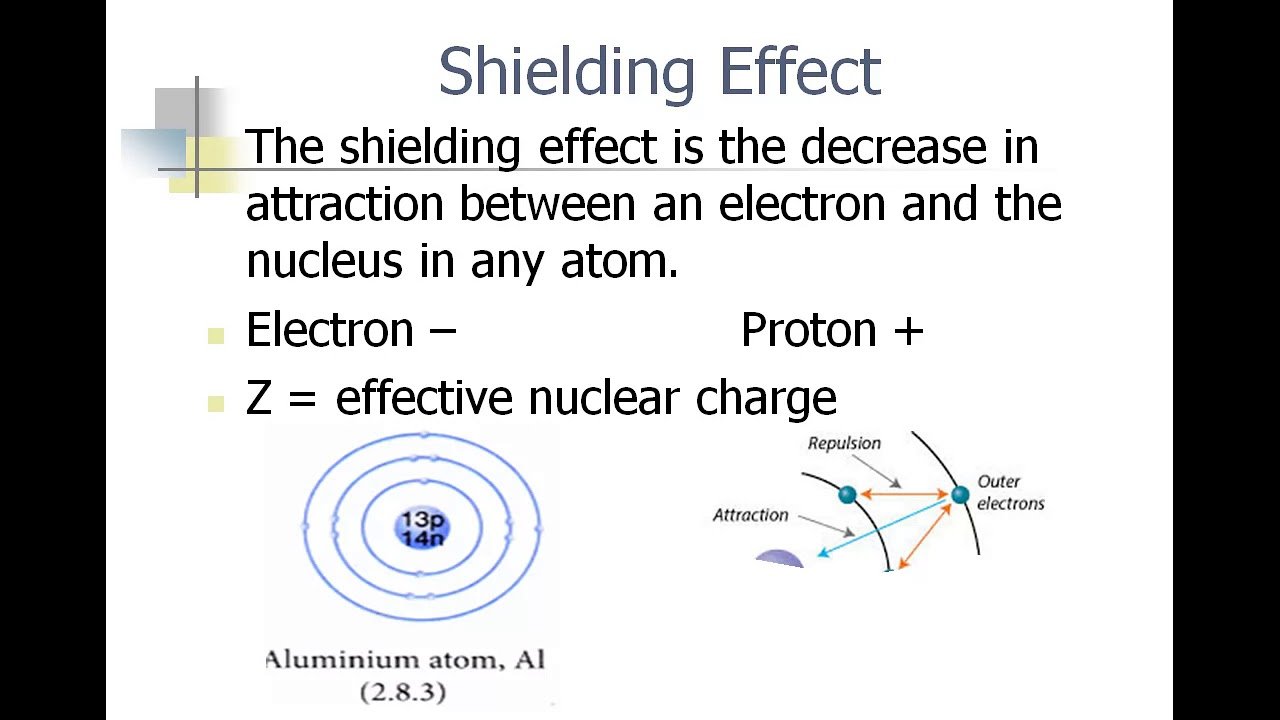
earth metals more reactive as you move down
More shielding halogens more reactive as you move up
Explanation:
The shielding effect is the electrons between the nucleus and the valence electrons acting as a âshieldââ repelling the outer electrons because they have the same charge, lowering the effective nuclear charge.
You can calculate the effective nuclear charge by
#Z_ = Z â S#
is the atomic number and #S# is the shielding electrons.
The more shielding electrons you have, the lower the ENC, so the less force there is holding onto the outer shell electrons.
If there is less force holding onto valence electrons, then they will be lost more easily, and likewise not gained as easily. Therefore when you move down the left-hand-side of the periodic table, atoms become more reactive â more liable to lost electrons. As you move down the right, however, atoms cannot gain electrons as easily, so as you move down the column of halogens, reactivity decreases.
This is because the left of the table lose electrons to form positive ions, while the right gains electrons to form negative ions.
Also, the lower the ENC due to greater shielding effect, the less electronegative an element will be, so whatever electrons it does have will not be pulled towards it so easily.
You May Like: What Does Math.floor Do In Java
Screening Effect Or Shielding Effect
In a multielectron atom, the valence shells electrons are attracted to the nucleus, and these electrons are repelled by the electrons present in the inner shells. On account of this, the actual force of attraction between the nucleus and the valence electrons is somewhat decreased by the repulsive forces acting in opposite directions. This decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is called screening effect or shielding effect.
The magnitude of the screening effect depends upon the number of inner electrons higher the number of inner electrons greater shall be the value of the screening effect. The symbol represents the screening effect constant.
Was this answer helpful?
Presentation On Theme: Shielding Effect The Shielding Effect Is The Reduction Of Attractive Force Between The Nucleus And Its Outer Electrons Due To The Blocking Affect Presentation Transcript:
1 Shielding EffectThe shielding effect is the reduction of attractive force between the nucleus and its outer electrons due to the blocking affect of the inner electronsNucleusShielding electronsValence electrons shielded by inner electrons
2 Shielding Effect Across stays the sameElectrons are added in the Valence shell and the shielding electrons remain the same
3 Shielding Effect Down increasesAnother layer of electrons is added and the shielding between the valence shell and the nucleus increases
4 Atomic RadiusOne half the distance from center to center of like atoms1/2
5 Atomic Radius Across decreasesAs the number of p+ in the nucleus and e- in the valence shell increases, the nucleus exerts a greater pull on all of the electrons
6 Atomic Radius Down increasesA new energy level is added increasing the size of the atoms
7 Ionization energyThe amount of energy needed to remove an electron1st Ionization Energy energy needed to remove the 1st e- from an atom+ IonizationEnergy++ e-
8 Ionization Energy Across increasese- are more strongly attracted to nucleus, increasing the energy necessary to remove an e-
9 Ionization Energy Down Decreasese- are further from the nucleus. Less energy is needed to remove an electron.
11 Ionic RadiusCations will be smaller than the original atom. This is due to a stronger nuclear pull.Anions will be larger than the original atom. The additional e- goes into the same energy level and the e- will repel each other and spread out.
You May Like: Is Paris Jackson Biologically Related To Michael
Shielding Effect Of D Electrons
In transition elements shielding effect is observed . Due to this zinc shows abnormality in atomic size.But at the same time we also say that gallium has same similar size as aluminium due to poor shielding effect of d electrons . Why is it that d electrons have good shielding effect in case of zinc but poor shielding effect in case of gallium?
When we are talking about zinc… We have to think about period trends. So in periods along moving from left to right covalent radius decreases. But in case of transition metal..
Two effects works first is $Z_$. Another effect is electronic repulsion.At the starting the no of electron in $d-$ orbital are less so $Z_$ is dominant over electronic repulsion but as we are moving alosng d-orbital electronic effect become dominant factor over $Z_$, So in the middle redius become almost constant and at the end it increases.
When we are talking about gallium and Aluminium radius comparision thats mean we are talking about group trends ,So in group trend abnormal size is due to poor shielding effect and relativistic effect. Normal trend on going down the size increases from aluminium to gallium size decreases because here a poor shielding of 3-d orbital present so $Z_$ penetarate and valence shell contract so size decreases.
What Is Shielding And Who Needs To Do It
People deemed most at risk of becoming seriously ill from the new coronavirus have been advised to shield by the government, meaning they should not leave their homes and should minimise all face-to-face contact until at least the end of June.
However, there has been some confusion about who exactly is required to shield and what this means in practice. New guidance that applies from 1 June permits people who are shielding to go outside in certain situations. Our readers have asked us to explain this.
You May Like: Beththomas
Effect Of Methyl Group Proximity On 17o Shielding Constant
After rationalizing this deshielding effect on the nuclear magnetic shielding constant, it is interesting to find out whether a similar effect is observed when a methyl group is spatially close to an oxygen atom. However, it can be expected that such an effect on 17O chemical shifts should depend strongly on the coordination state of that atom. Some time ago, it was observed experimentally that a methyl group close to a carbonyl oxygen atom produces a 17O shielding effect of about 12.0 ppm , where 17O chemical shifts were measured, under the same conditions, for formamide, 9, and for cis–N-methyl formamide, 10. It is expected that the syn and anti orientations of the methyl group should yield different effects on the nuclear magnetic shielding constant. In Table 10, several molecular parameters are compared for syn and anti conformations of the methyl group in 10.
Table 10. Comparison of Some Properties for the Methyl Group Conformations syn and anti in N-Methyl Formamide
| Property |
|---|
- Diamagnetic contribution: 444.7 ppm paramagnetic contribution: 446.6 ppm.
- c
- Experimental value + 12.0 ppm of the shielding effect for the difference on in 10 and 9, Ref. .
Although the + 13.8 ppm difference on for syn and anti orientations is determined from both the diamagnetic and paramagnetic contributions, the latter dominates that trend.
Jógvan Magnus Haugaard Olsen, Jacob Kongsted, in, 2011
The Structure Of The C
The structure of the C– and N-trimethylsilylpyrazoles , the effect of substituents on the shielding constants of the magnetically active nuclei of the pyrazole ring and silylotropic transformation of 4-substituted N-trimethylsilylpyrazoles has been studied by multinuclear NMR spectroscopy . We first studied the stereochemical structure and silylotropic rearrangement of the trimethylsilyl azole derivatives in detail.
Scheme 7. The silylotropic rearrangement in 4-substituted N-trimethylsilylpyrazoles.
The parameters of 1H, 13C and 29Si NMR spectra of C– and N-TMS-pyrazoles are shown in Tables 3 and 4 . The resonance position of proton signals of the C-trimethylsilyl group in the 1H NMR spectra varies in the range 0.20.4 ppm, while proton signals of the N-trimethylsilyl groupin the range of 0.400.46 ppm .
Table 3. 1H, 13C and 29Si NMR chemical shifts of mono-, bis– and tris pyrazoles .
| 1 |
|---|
- = .
Scheme 8. The structure of C– and N-trimethylsilylpyrazoles.
The chemical shifts of the ring protons of the heterocycle practically do not change with the introduction of the Me3Si-group and only weakly depend on its location in the pyrazole cycle.
Scheme 9. The prototropic exchange in 3-substituted pyrazoles.
The thermodynamic constant of a tautomeric equilibrium is calculated from Eq. :
where Tctemperature of coalescence of NMR signals, difference between NMR signals at low temperature in the condition of the limiting slow exchange.
| Compound |
|---|
Also Check: Who Is Paris Jackson Parents
What Is The Goal Of A Roller Derby Game
Roller derby is a popular sport, although it is unfamiliar to many people. The basic purpose is to set one team member past the opposing team to score points. Other members of the team serve as blockers to prevent the opposing team from stopping the jammer. Blockers interfere with the interaction between the jammer and the opponents by getting between the jammer and the skaters trying to stop her.
The attraction between an electron and the nucleus of the atom is not a simple issue. Only with hydrogen is there a one-to-one relationship that can be discussed in terms of direct charge attraction. As the size of the atom increases, the number of protons and electrons also increase. These changes influence how the nucleus attracts electrons.
In general, the ionization energy of an atom will increase as we move from left to right across the periodic table. There are several exceptions to the general increase in ionization energy across a period. The elements of Group 13 have lower ionization energies than the elements of Group 2 . This is an illustration of a concept called electron shielding . Outer electrons are partially shielded from the attractive force of the protons in the nucleus by inner electrons.
Figure 1. The shielding effect is shown by the interior electron cloud shielding the outer electron of interest from the full attractive force of the nucleus. A larger shielding effect results in a decrease in ionization energy.
What Is The Difference Between Shielding And Screening Effect
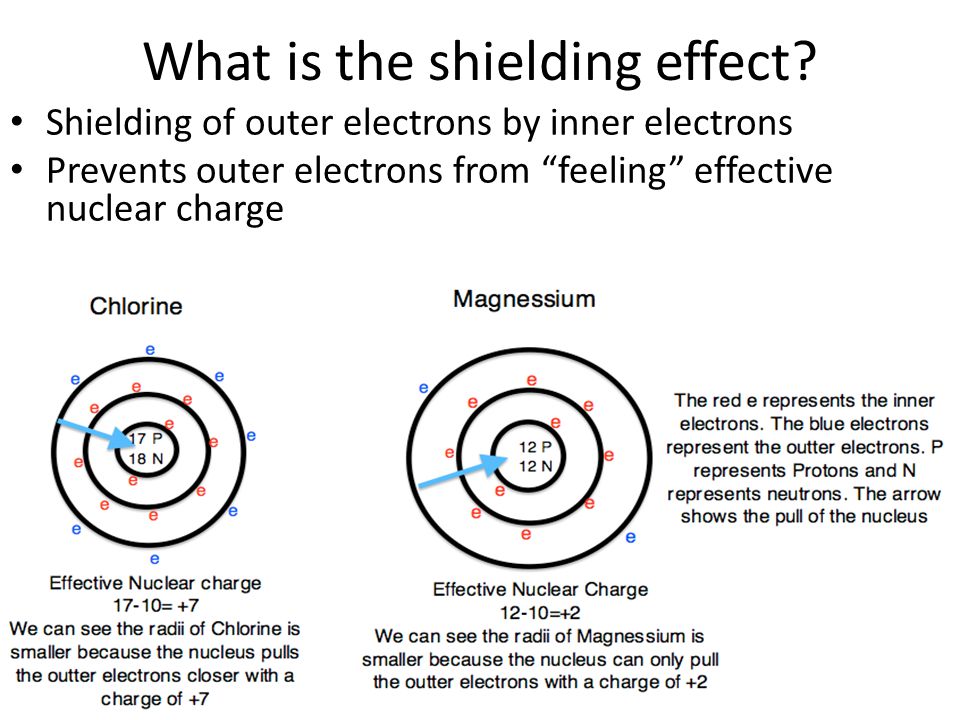
- Shielding effect is the reduction in the effective nuclear charge on the electron cloud, due to differences in the attraction forces between electrons and the nucleus. Shielding effect is also known as the Screening Effect. Hence, there is no difference between these two terms. They primarily mean the same thing.
You May Like: Cci4 Lewis Structure
What Influences Atomic Radius
The bigger the atomic number, the larger the atoms radius. This is especially true as you move straight down a given column on the periodic table the radius of each successive neighboring atom increases. The growing size is due to the increasing number of filled electron shells as you move down the periodic table.
What Is Effective Nuclear Charge
Effective nuclear change means the net positive charge which affects the attraction of outer electron particles from the nucleus of a polyelectronic atom. This term is used because the shielding electrons prevent the attraction of the outer orbital electron of an atom. Therefore, the effective nuclear charge calculating from Slaters rule = Z , where = shielding or screening constant.
You May Like: Holt Geometry Worksheets
Calculating The Effective Nuclear Charge:
An estimate of effective nuclear charge can be obtained from Zeff = Z â S, where Zeff = effective nuclear charge, Z = atomic number, and, S = the screening constant.ââConsider aluminum: 3s23p1 ââZ = 13 S = 10 Zeff = Z â S = 13 â 10 = 3+
Dont forget that Zeff is only an estimate. Actual shielding effect is always greater that the screening constant S because core electrons are much closer to the nucleus than are valence electrons.
Considering Shielding Effects Is Essential For Modelling Metalligand Interactions Accurately
Fig. 5151342515
Understanding how metalligand interactions affect ligand binding affinities has important ramifications in the design of potent metal-bound ligands. It is therefore vital for developing reliable scoring functions. It is impossible to develop accurate scoring functions without correctly modelling metalligand interactions. Given that the catalytic power of a metalloenzyme is the difference in binding free energy between the substrate in the TS and the substrate in the GS , this study also provided a novel approach for exploring the enormous catalytic power of the metal ions in metalloenzymes.
Read Also: What Math Class Do 9th Graders Take
What Is The Shielding Effect
In chemistry, the shielding effect is the weakening of the attraction between an electron and an atomic nucleus with more than one electron shell. The effect is also called screening or atomic shielding.
In atoms and ions with only one electron, the total force experienced by an orbiting electron is equal to the electromagnetic attractive force that the nucleus exerts on this electron. When there are more electrons orbiting the nucleus, each electron experiences this nuclear electromagnetic attraction in addition to repulsion forces by the surrounding electrons. The magnitude of this repulsive force depends on the number of electrons, so as the number of filled electron shells increases, the net force on the outermost electrons decreases. These outer shell electrons are not as strongly bonded to the nucleus as the electrons in inner shells, explaining why valence-shell electrons are more easily removed from an atom than inner shell ones.
A larger number of orbiting electrons results in more complex repulsive interactions between these electrons, making the quantitative assessment of the repulsive force resulting from the shielding effect difficult. Techniques to determine the shielding effect include numerical solutions of the Schrodinger wave equation, using Slater empirical formulas or inferring the effect using the Rutherford backscattering spectrometry.
Recommended Reading: Fsa Algebra 1 Eoc Practice Test Answers