Ph Definition And Formula
pH is a measure of hydrogen ion concentration , which in turn is a gauge of how acidic or basic a chemical solution is. Ordinarily, the pH scale runs from 0 to 14, although its actually possible to get negative values and ones exceeding 14. A pH value around 7 is neutral , a pH value less than 7 is acidic, and a pH value greater than 7 is basic. The pH value tells whether a chemical is an acid or a base, but it doesnt indicate the strength of the acid or base. pH is related to pOH, which is the power of the hydroxide ion and is used when discussing bases. The formulas to calculate pH and pOH are:pH = log
pKa = log Kaat half the equivalence point, pH = pKa = -log Ka
A large Ka value indicates a strong acid because it means an acid largely dissociates into its ions. A large Ka value also means the reaction arrow favors the formation of production. In contrast, a small Ka value means only a small amount of acid dissociates, indicating a weak acid. A small Ka value means the reaction favors the reactants rather than the products. Most weak acids have Ka values between 10-2 to 10-14.
pKa gives the same information, but in a different way. The smaller the pKa value, the stronger the acid. Or, the larger the pKa value, the weaker the acid. Weak acids typically have pKa values between 2 and 14.
Equilibrium Constants Ka And Kb: Pka Pkb
The acid dissociation constant is a quantitative measure of the strength of an acid in solution while the base dissociation constant is a measure of basicitythe bases general strength.
Ka and pKaAcids are classified as either strong or weak, based on their ionization in water. A strong acid is an acid which is completely ionized in an aqueous solution. A weak acid is an acid that ionizes only slightly in an aqueous solution.
The ionization for a general weak acid, HA, can be written as follows:
Because the acid is weak, an equilibrium expression can be written. An acid ionization constant is the equilibrium constant for the ionization of an acid.
The acid ionization represents the fraction of the original acid that has been ionized in solution. Therefore, the numerical value of Ka is a reflection of the strength of the acid. Weak acids with relatively higher Ka values are stronger than acids with relatively lower Ka values. Because strong acids are essentially 100% ionized, the concentration of the acid in the denominator is nearly zero and the Ka value approaches infinity. For this reason, Ka values are generally reported for weak acids only.
The logarithmic constant is equal to -log10. The larger the value of pKa, the smaller the extent of dissociation. A weak acid has a pKa value in the approximate range of -2 to 12 in water. Acids with a pKa value of less than about -2 are said to be strong acids.
Practice Questions
Solutions Of Strong Acids And Bases: The Leveling Effect
You will notice in Table \ that acids like \ and \ lie above the hydronium ion, meaning that they have \ values less than zero and are stronger acids than the \ ion. Recall from Chapter 4 that the acidic proton in virtually all oxoacids is bonded to one of the oxygen atoms of the oxoanion. Thus nitric acid should properly be written as \. Unfortunately, however, the formulas of oxoacids are almost always written with hydrogen on the left and oxygen on the right, giving \ instead. In fact, all six of the common strong acids that we first encountered in Chapter 4 have \ values less than zero, which means that they have a greater tendency to lose a proton than does the \ ion. Conversely, the conjugate bases of these strong acids are weaker bases than water. Consequently, the proton-transfer equilibria for these strong acids lie far to the right, and adding any of the common strong acids to water results in an essentially stoichiometric reaction of the acid with water to form a solution of the \ ion and the conjugate base of the acid.
In aqueous solutions, \ is the strongest acid and \ is the strongest base that can exist in equilibrium with \.
Other examples that you may encounter are potassium hydride ) and organometallic compounds such as methyl lithium ).
Read Also: Beth Thomas Child Of Rage Today
What Is Pka In Organic Chemistry
4.2/5pKapKapKapKa
Simply so, what does the pKa of a functional group tell you?
pKa does not show if a molecule is basic or acidic. Basic molecules gain protons and acidic molecules donate protons. If a molecule is a base or an acid, depends on their functional groups. It can only tell you how strong the base or acid is.
Similarly, what does high pKa mean? A lower pKa means the Ka value is higher and a higher Ka value means the acid dissociates more readily because it has a larger concentration of Hydronium ions .
In this way, what is the pKa of alcohol?
| Group |
|---|
| 17.2 |
What does Ka and pKa mean?
A large Ka value indicates a strong acid because it means the acid is largely dissociated into its ions. A large Ka value also means the formation of products in the reaction is favored. The smaller the value of pKa, the stronger the acid. Weak acids have a pKa ranging from 2-14.
Examples Of Acid Dissociation Constants Ka For A Strong Acid A Weak Acid And An Extremely Weak Acid
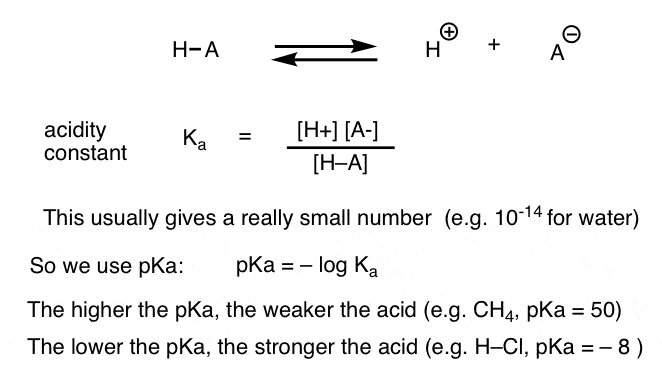
Lets look at 3 examples. Note that these are somewhat oversimplified in that the solvent has been left out.*
Now comparing acidity between numbers with lots of exponents after them is not the most convenient way to do things. So instead, weve taken to using a logarithmic scale. These are common the Richter scale is logarithmic, for instance an 8.0 magnitude quake is 10 times more powerful than a 7.0 magnitude quake.
Read Also: Holt Geometry Chapter 7
What Does The P Mean
Whenever you see a “p” in front of a value, like pH, pKa, and pKb, it means you’re dealing with a -log of the value following the “p”. For example, pKa is the -log of Ka. Because of the way the log function works, a smaller pKa means a larger Ka. pH is the -log of hydrogen ion concentration, and so on.
Dissociation Constant Of Water
The dissociation constant of water is denoted Kw:
- K }=}
The concentration of water O }} is omitted by convention, which means that the value of Kw differs from the value of Keq that would be computed using that concentration.
The value of Kw varies with temperature, as shown in the table below. This variation must be taken into account when making precise measurements of quantities such as pH.
| Water temperature |
|---|
Also Check: Klohe Kardashians Real Father
What Does Ka Stand For Chemistry
We compiled queries of the KA abbreviation in Chemistry in search engines. The most frequently asked KA acronym questions for Chemistry were selected and included on the site.
We thought you asked a similar KA question to the search engine to find the meaning of the KA full form in Chemistry, and we are sure that the following Chemistry KA query list will catch your attention.
Molecules With One Binding Site
Experimentally, the concentration of the molecule complex is obtained indirectly from the measurement of the concentration of a free molecules, either or .In principle, the total amounts of molecule 0 and 0 added to the reaction are known.They separate into free and bound components according to the mass conservation principle:
- }& =+ }}\\}& =+ }}\end}}
To track the concentration of the complex , one substitutes the concentration of the free molecules , of the respective conservation equations, by the definition of the dissociation constant,
- =K_}+}
This yields the concentration of the complex related to the concentration of either one of the free molecules
- }=}}+}}=}}+}}}
Read Also: Holt Geometry Chapter 7 Test
What Is Ka And Pka In Chemistry
4/5KapKaKaKapKathis is here
Key Takeaways: pKa DefinitionThe pKa value is one method used to indicate the strength of an acid. pKa is the negative log of the acid dissociation constant or Ka value. A lower pKa value indicates a stronger acid. That is, the lower value indicates the acid more fully dissociates in water.
Also Know, how does KA relate to pH? Note that x is Related to pH and Calculate KaSince x = and you know the pH of the solution, you can write x = 10-2.4. It is now possible to find a numerical value for Ka. Ka = 2 / = 1.8 x 10-5.
Also to know, how do you get pKa from Ka?
To create a more manageable number, chemists define the pKa value as the negative logarithm of the Ka value: pKa = -log Ka. If you already know the pKa value for an acid and you need the Ka value, you find it by taking the antilog. In practice, this means raising both sides of the equality to exponents of 10.
Why is pKa important?
pH and pKaThe lower the pH, the higher the concentration of hydrogen ions . The lower the pKa, the stronger the acid and the greater its ability to donate protons. This is important because it means a weak acid could actually have a lower pH than a diluted strong acid.
Cumulative And Stepwise Constants
A cumulative equilibrium constant, denoted by , is related to the product of stepwise constants, denoted by K . For a dibasic acid the relationship between stepwise and overall constants is as follows
- H
- =\rho }}}
and since the molar mass M is a constant in dilute solutions, an equilibrium constant value determined using will be simply proportional to the values obtained with and .
It is common practice in biochemistry to quote a value with a dimension as, for example, “Ka = 30 mM” in order to indicate the scale, millimolar or micromolar of the concentration values used for its calculation.
Recommended Reading: Molecular Geometry Ccl4
The Pka Scale Encompasses Over Sixty Orders Of Magnitude
That scale comprises 60 orders of magnitude. Thats a huge number!!!!!
To give you an idea of the scale, of pKa, this is the range of the smallest value for length , to the width of the known universe . This website has a phenomenal animation of this.
Anyhow, these measurements have been done for thousands of different molecules now. The result is a big table that allows us to compare the acidity of all kinds of different functional groups. Heres an example of a pKa table from a previous post.
The pKa table is your friend. In one document, it gives you information on the scope and magnitude of a wide range of chemical behavior the strongest of the strong acids, and the weakest of the weak acids. And since the stronger the acid, the weaker the conjugate base, it also provides information about basicity.
Next Post: How to Use A pKa Table
What Is The Meaning Of Ka Abbreviation In Chemistry
What is KA definition ?
KA definition is “Acid Dissociation Constant”.
What does KA mean in Chemistry?
KA mean that “Acid Dissociation Constant” for Chemistry.
What is KA acronym ?
KA acronym is “Alkaline Phosphatase”.
What is shorthand of Acid Dissociation Constant ?
The shorthand of “Acid Dissociation Constant” is KA.
What is the definition of KA acronym in Chemistry?
Definitions of KA shorthand is “Kinetic Atom”.
What is the full form of KA abbreviation?
Full form of KA abbreviation is “Kinetic Atom”.
What is the full meaning of KA in Chemistry?
Full meaning of KA is “Alkaline Phosphatase”.
What is the explanation for KA in Chemistry?
Explanation for KA is “Acid Dissociation Constant”.
What is the meaning of KA Abbreviation in Astrology ?
The site does not only include the meanings of the KA abbreviation in Chemistry. Yes, we know your main purpose is explanation of KA abbreviation in Chemistry. However, we thought that besides the meaning of the KA definitions in Chemistry, you can consider astrological information of KA acronym in Astrology. Therefore, the astrological explanation of each word in each KA abbreviation is also included.
KA Abbreviation in Astrology
Recommended Reading: Beth Thomas Psychopathic Child
What Are The Units Of Kd
Kd is the equilibrium constantequilibrium constantEvery chemical equilibrium can be characterized by an equilibrium constant, known as K eq. The K eq and K P expressions are formulated as amounts of products divided by amounts of reactants each amount is raised to the power of its coefficient in the balanced chemical equation.opentextbc.ca chapter the-equilibrium-constant-2The Equilibrium Constant Introductory Chemistry 1st Canadian for the dissociationdissociationDissociation in chemistry and biochemistry is a general process in which molecules separate or split into smaller particles such as atoms, ions, or radicals, usually in a reversible manner. Dissociation is the opposite of association or recombination.en.wikipedia.org wiki Dissociation_Dissociation Wikipedia equi- librium, it is equal to Kon/Koff, and its units are M. It should not be confused with Koff, which is the rate constantrate constantA constant of proportionality relating the rate of a chemical reaction to the concentrations of the chemical species involved in the reaction. For a second-order reaction, A + B products, the units of the rate coefficient are -1-1.glossary.ametsoc.org wiki Rate_coefficientRate coefficient Glossary of Meteorology AMS Glossary for the breaking of the complex.
Acid Dissociation Constant From Ph
The acid dissociation constant may be found it the pH is known. For example:
Calculate the acid dissociation constant Ka for a 0.2 M aqueous solution of propionic acid that is found to have a pH value of 4.88.
To solve the problem, first, write the chemical equation for the reaction. You should be able to recognize propionic acid is a weak acid . It’s dissociation in water is:
CH3CH2CO2H + H2 H3O++ CH3CH2CO2-
Set up a table to keep track of the initial conditions, change in conditions, and equilibrium concentration of the species. This is sometimes called an ICE table:
Read Also: Is Paris Jackson Michael’s Biological Daughter
What Does Ka Stand For
What does KA mean? This page is about the various possible meanings of the acronym, abbreviation, shorthand or slang term: KA.
Filter by:
Popularity rank for the KA initials by frequency of use:
Couldn’t find the full form or full meaning of KA?
Maybe you were looking for one of these abbreviations:
Discuss these KA abbreviations with the community:
Report Comment
We’re doing our best to make sure our content is useful, accurate and safe.If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we’ll take care of it shortly.
Values For Common Substances
There are multiple techniques to determine the pKa of a chemical, leading to some discrepancies between different sources. Well measured values are typically within 0.1 units of each other. Data presented here were taken at 25 °C in water. More values can be found in the Thermodynamics section, above. A table of pKa of carbon acids, measured in DMSO, can be found on the page on carbanions.
| Chemical |
|---|
Also Check: Khloes Real Father
Pka And Dissociation Equilibrium
Acids include strong acids, which completely dissociate in water, and weak acids, which only partially dissociate. When an acid dissociates, it releases a proton to make the solution acidic, but weak acids have both a dissociated state and undissociated state that coexist according to the following dissociation equilibrium equation.
The concentration ratio of both sides is constant given fixed analytical conditions and is referred to as the acid dissociation constant . Ka is defined by the following equation.
The square brackets indicate the concentration of respective components. Based on this equation, Ka expresses how easily the acid releases a proton . In addition, the equation shows how the dissociation state of weak acids vary according to the level in the solution.Carboxylic acids , such as acetic and lactic acids, normally have a Ka constant of about 10-3 to 10-6. Consequently, expressing acidity in terms of the Ka constant alone can be inconvenient and not very intuitive.Therefore, pKa was introduced as an index to express the acidity of weak acids, where pKa is defined as follows.
For example, the Ka constant for acetic acid is 0.0000158 , but the pKa constant is 4.8, which is a simpler expression. In addition, the smaller the pKa value, the stronger the acid. For example, the pKa value of lactic acid is about 3.8, so that means lactic acid is a stronger acid than acetic acid.
Calculating A Ka Value From A Known Ph
The quantity pH, or “power of hydrogen,” is a numerical representation of the acidity or basicity of a solution. It can be used to calculate the concentration of hydrogen ions or hydronium ions in an aqueous solution. Solutions with low pH are the most acidic, and solutions with high pH are most basic.
Read Also: 4 Goals Of Psychology Example