Erin Brockovich And Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr used by Pacific Gas & Electric to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brokovich , Erin and lawyer Edward Masry sued PG& E for contaminating the water near Hinckley in 1993. The settlement they won in 1996$333 millionwas the largest amount ever awarded for a direct-action lawsuit in the US at that time.
Figure 1.
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr or Cr forms. Cr, an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin.
What Does The Per Mean In Chemistry
ChemistryprefixPart 1Writing Chemical Formulas of Covalent Compounds
Naming Binary Covalent Compounds
There are many other naming schemes. Thereare naming schemes for acids, organic compounds and simple covalent compounds. You book covers simple covalent compounds in this chapter probablybecause it is so similar to the naming scheme for ionic compounds. Remember, ionic compounds are metal combined with a non-metal. A covalent compound is the combination of non-metals.
Rules for naming simple covalentcompounds:
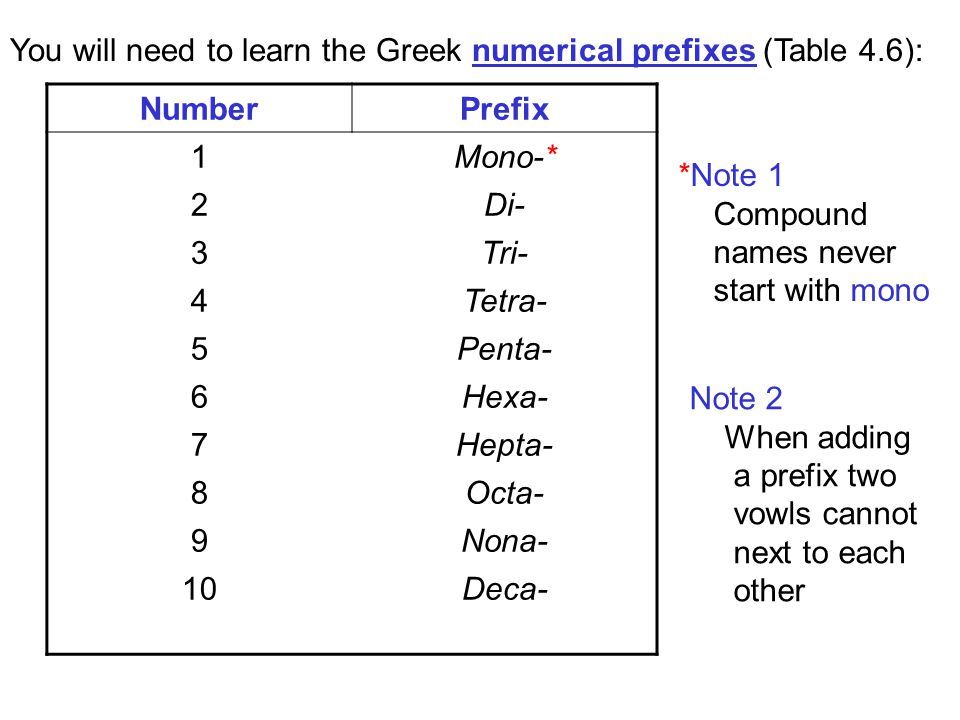
1. Name the non-metal furthest to the left on the periodic tableby its elemental name.
2. Name the other non-metal by its elemental name and an -ideending.
3. Use the prefixes mono-, di-, tri-…. to indicate the numberof that element in the molecule.
4. If mono is the first prefix, it is understood and not written
N2O4 iscalled dinitrogen monoxide
CO2 is called carbondioxide
CO is called carbon monoxide
N2O is called dinitrogenmonoxide.
CCl4 is called carbontetrachloride
Here is a chart of those prefixes:
|
1 – mono |
Read Also: Elton John Children Biological
What Are The Prefixes In Organic Chemistry
The ones that students confuse all the time are below:
Methane – 1 carbon compound
For compounds with 5-10 carbons, add the Greek prefix to “-ane”.
For example,
Get a free answer to a quick problem. Most questions answered within 4 hours.
OR
Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.
Why Do We Use Prefixes In Chemistry
When naming molecular compounds prefixes are used to dictate the number of a given element present in the compound. mono- indicates one, di- indicates two, tri- is three, tetra- is four, penta- is five, and hexa- is six, hepta- is seven, octo- is eight, nona- is nine, and deca is ten.
You May Like: Lewis Dot Structure For Ccl4
How Do You Name A Company
Here are 12 helpful suggestions on how to come up with a winning name for your business:
Iupac Nomenclature Of Organic Chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry . It is published in the Nomenclature of Organic Chemistry . Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantially shorter and clearer, and so preferred. These non-systematic names are often derived from an original source of the compound. Also, very long names may be less clear than structural formulas.
You May Like: My Hrw Com Algebra 1
Common Acid And Anion Names
Acids are compounds containing an ionizable proton , since an acid is a proton donor . The polyatomic anions derived from acids are named by dropping the-ic suffix from the acid name and adding the-ate suffix, respectively. Compounds containing polyatomic anions are named using the Type I or Type II naming systems described above. For example, the sodium salt of nitric acid is sodium nitrate . If you know the acid formula you will alwaysget the correct anion formula and its charge, since the charge is equal to the number of ionizable hydrogen atoms in the acid, and is always negative. For example, for sulfuric acid , the anion is sulfate with a -2 charge.
Acids which do not contain oxygen are named by adding the hydro- prefix to the root name of the element, followed by the -ic suffix. HCl ishydrochloric acid, H2S ishydrosulfuric acid, and HF is hydrofluoric acid . Anions of these acids, which contain a single element , are named as a regular non-metal anion .
| Acid |
|---|
Naming Acids And Bases
Read Also: Who Is Paris Jackson Mother
What Are The Prefixes For Molecular Compounds
What are the prefixes for molecular compounds?
Here are the prefixes in naming molecular compounds:
Mono- 1
Nona- 9
Molecular compounds are named using a systematic approach of prefixes to indicate the number of each element present in the compound.
Ihopeithelps~
Answer:
In nomenclature of simple molecular compounds, the more electropositive atom is written first and the more electronegative element is written last with an -ide suffix.
The Greek prefixes are used to dictate the number of a given element present in a molecular compound.
Prefixes can be shortened when the ending vowel of the prefix conflicts with a starting vowel in the compound.
Common exceptions exist for naming molecular compounds, where trivial or common names are used instead of systematic names, such as ammonia instead of nitrogen trihydride or water instead of dihydrogen monooxide.
Terms
nomenclatureA set of rules used for forming the names or terms in a particular field of arts or sciences.
electronegativeTending to attract electrons within a chemical bond.
electropositiveTending to not attract electrons within a chemical bond.
Chemical Nomenclature
Molecular compounds are made when two or more elements share electrons in a covalent bond to connect the elements. Typically, non-metals tend to share electrons, make covalent bonds, and thus, form molecular compounds.
Rules for Naming Molecular Compounds:
Remove the ending of the second element, and add ide just like in ionic compounds.
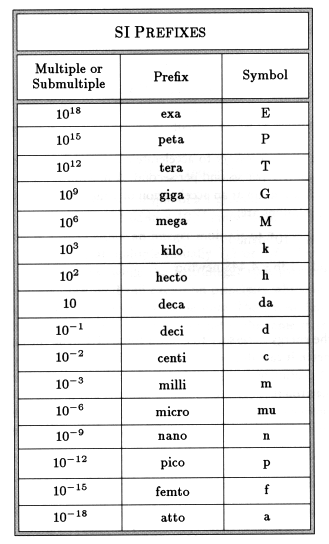
Examples Using Metric Prefixes
The distance from City A to City B is 8.0 x 103 meters. From the table, 103 can be replaced with the prefix ‘kilo’. Now the distance could be stated as 8.0 kilometers or shortened further to 8.0 km.
The distance from Earth to the Sun is approximately 150,000,000,000 meters. You could write this as 150 x 109 m, 150 gigameters or 150 Gm.
The width of human hair runs on the order of 0.000005 meters. Rewrite this as 50 x 10-6m, 50 micrometers, or 50 m.
Read Also: Figure Definition Psychology
The Old Classic Or Common Way Of Naming
Names of some ionic compounds: Common, or trivial, names of compounds are sometimes used in informal conversations between chemists, especially older chemists. Systematic names are formal names that are always used in print.
Since some metallic elements form cations that have different positive charges, the names of ionic compounds derived from these elements must contain some indication of the cation charge. The older method uses the suffixes -ous and -ic to denote the lower and higher charges, respectively. In the cases of iron and copper, the Latin names of the elements are used . This system is still used, although it has been officially supplanted by the more precise, if slightly cumbersome, Stock system. In both systems, the name of the anion ends in -ide.
Naming Compounds Part 1 YouTube: This video explains how to name covalent and ionic compounds.
Interesting Metric Prefix Trivia

Not all of the metric prefixes that were proposed were adopted. For example, myria- or myrio- and the binary prefixes double- and demi- were originally used in France in 1795, but were dropped in 1960 because they were not symmetrical or decimal.
The prefix hella- was proposed in 2010 by UC Davis student Austin Sendek for one octillion . Despite receiving significant support, the Consultative Committee for Units rejected the proposal. Some websites did, however, adopt the prefix, notably Wolfram Alpha and Google Calculator.
Because the prefixes are based on units of ten, you don’t have to use a calculator to perform conversions between different units. All you need to do is move the decimal point to the left or right or add/subtract exponents of 10 in scientific notation.
For example, if you want to convert millimeters to meters, you can move the decimal point three places to the left: 300 millimeters = 0.3 meters
If you have trouble trying to decide which direction to move a decimal point, use common sense. Millimeters are small units, while a meter is large , so there should be lots of millimeters in a meter.
Converting from a large unit to a smaller unit works the same way. For example, converting kilograms to centigrams, you move the decimal point 5 places to the right : 0.040 kg = 400 cg
Read Also: Geometry Segment Addition Postulate Worksheet
What Is Prefix And Example
A prefix is an affix which is placed before the stem of a word. Adding it to the beginning of one word changes it into another word. For example, when the prefix un- is added to the word happy, it creates the word unhappy. In English, there are no inflectional prefixes English uses suffixes instead for that purpose.
What Are All The Prefixes In Chemistry
When naming molecular compounds prefixes are used to dictate the number of a given element present in the compound. mono- indicates one, di- indicates two, tri- is three, tetra- is four, penta- is five, and hexa- is six, hepta- is seven, octo- is eight, nona- is nine, and deca is ten.
Read Also: Holt Mcdougal Geometry Answers And Work
Key Concepts And Summary
Chemists use nomenclature rules to clearly name compounds. Ionic and molecular compounds are named using somewhat-different methods. Binary ionic compounds typically consist of a metal and a nonmetal. The name of the metal is written first, followed by the name of the nonmetal with its ending changed to ide. For example, K2O is called potassium oxide. If the metal can form ions with different charges, a Roman numeral in parentheses follows the name of the metal to specify its charge. Thus, FeCl2 is iron chloride and FeCl3 is iron chloride. Some compounds contain polyatomic ions the names of common polyatomic ions should be memorized. Molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a molecule of the compound. Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. Acids are an important class of compounds containing hydrogen and having special nomenclature rules. Binary acids are named using the prefix hydro-, changing the ide suffix to ic, and adding acid HCl is hydrochloric acid. Oxyacids are named by changing the ending of the anion to ic, and adding acid H2CO3 is carbonic acid.
Compounds Composed Of Two Elements
When two nonmetallic elements form a molecular compound, several combination ratios are often possible. For example, carbon and oxygen can form the compounds CO and CO2. Since these are different substances with different properties, they cannot both have the same name . To deal with this situation, we use a naming method that is somewhat similar to that used for ionic compounds, but with added prefixes to specify the numbers of atoms of each element. The name of the more metallic element is first, followed by the name of the more nonmetallic element with its ending changed to the suffix ide. The numbers of atoms of each element are designated by the Greek prefixes shown in Table 11.
| Number |
|---|
| Table 14. Names of Common Oxyacids |
You May Like: Geometry Segment Addition Postulate Worksheet
Prefixes Of Base Units By Factors Of Ten
Metric or SI units are based on units of ten. Very large or very small numbers are easier to work with when you can replace any scientific notation with a name or word. The metric unit prefixes are short words that indicate a multiple or fraction of a unit. The prefixes are the same no matter what the unit is, so decimeter means 1/10th of a meter and deciliter means 1/10th of a liter, while kilogram means 1000 grams and kilometer means 1000 meters.
Decimal-based prefixes have been used in all forms of the metric system, dating back to the 1790s. The prefixes used today have been standardized from 1960 to 1991 by the International Bureau of Weights and Measures for use in the metric system and the International System of Units .
When Are The Prefixes Bis Tris Etc Used In Nomenclature Of Complexs
For naming coordination compounds, the multiplicative prefixes di, tri, tetra, penta, etc., are used in the case of simple ligands, for example:
$\ce$ tetrachloridoplatinate
The alternative multiplicative prefixes bis, tris, tetrakis, pentakis, etc. are not only used for bidentate ligands. They are generally used with composite ligand names or in order to avoid ambiguity, for example:
$\ce$ tetrakisplatinum
The corresponding section in the current version of Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 reads as follows:
IR-9.2.2.2 Number of ligands in a coordination entity
Two kinds of multiplicative prefix are available for indicating the number of each type of ligand within the name of the coordination entity .
Prefixes di, tri, etc. are generally used with the names of simple ligands. Enclosing marks are not required.
Prefixes bis, tris, tetrakis, etc. are used with complex ligand names and in order to avoid ambiguity. Enclosing marks must be placed around the multiplicand.
The use of multiplicative prefixes is also explained in the IUPAC Technical Report Brief guide to the nomenclature of inorganic chemistry. Pure Appl. Chem.2015,87, 10391049 as well as in the corresponding four-sided lift-out document, which is available as supplementary material.
Composite ligand names include ligand names which are substituted or which themselves contain multiplicative prefixes or locants.
Read Also: Value Of Amu Degree
Order Of Precedence Of Group
When compounds contain more than one functional group, the order of precedence determines which groups are named with prefix or suffix forms. The table below shows common groups in decreasing order of precedence. The highest-precedence group takes the suffix, with all others taking the prefix form. However, double and triple bonds only take suffix form and are used with other suffixes.
Prefixed substituents are ordered alphabetically , e.g. chlorofluoromethane, not fluorochloromethane. If there are multiple functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically The N position indicator for amines and amides comes before “1”, e.g., CH3CHCH2NH is N,2-dimethylpropanamine.
Nomenclature Of Alkene Stereoisomers
Read Also: Does Kamala Harris Have Any Biological Children
The Stock Method Of Naming
An ionic compound is named first by its cation and then by its anion. The cation has the same name as its element. For example, K+1 is called the potassium ion, just as K is called the potassium atom. The anion is named by taking the elemental name, removing the ending, and adding -ide. For example, F-1 is called fluoride, for the elemental name, fluorine. The -ine was removed and replaced with -ide. To name a compound, the cation name and the anion named are added together. For example, NaF is also known as sodium fluoride.
If either the cation or the anion was a polyatomic ion, the polyatomic ion name is used in the name of the overall compound. The polyatomic ion name stays the same. For example, Ca2 is called calcium nitrate.
For cations that take on multiple charges , the charge is written using Roman numerals in parentheses immediately following the element name. For example, Cu2 is copper nitrate, because the charge of two nitrate ions is 2 = -2. Since the net charge of the ionic compound must be zero, the Cu ion has a 2+ charge. This compound is therefore, copper nitrate. The Roman numerals in fact show the oxidation number, but in simple ionic compounds this will always be the same as the metals ionic charge.