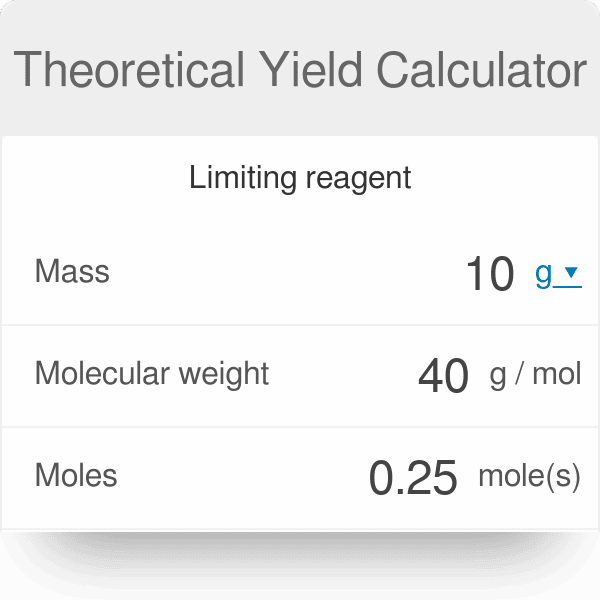
Calculate Molar Mass Of Reactants
Molar mass is the mass of one mole of a substance. It’s also known as molar weight and can be calculated using average atomic masses on the periodic table. Use the below atomic masses taken from the periodic table to calculate the molar mass of each reactant rounded to 1 decimal point.
|
Element |
- n = number of moles
In the example, we have 0.10L of nitrobenzene. Convert this to moles using a line equation using the given density .
We also have 0.30L of triethylene glycol. Convert this to moles with a line equation using the given density.
Calculate The Theoretical Yield
The third step in finding the percentage yield is to calculate the theoretical yield of chemical reaction. There are two main methods to find the theoretical yield of a reaction. The one used to find theoretical yield at the industrial level and the other that is used to calculate theoretical yield at more minor levels .
For this purpose, all you have to do is to multiply the number of moles with the molar mass of each reactant and then sum up all the values together, such as
2H2 + O2 – – – > 2H2O
2 + 2 = 36g
Thus, the theoretical yield of water produced by the reaction of two moles of hydrogen and one mole of oxygen will be 36g.
CO + 2H2 – – – > CH3OH
Suppose you have been asked to find the theoretical yield of methyl hydroxide produced by 1.2 tons of hydrogen gas. At the same time, the amount of CO left even after the completion of the reaction.
Related: Complete process of a metal displacement reaction in an aqueous medium with different examples.
It is clear from the problem that the limiting reactant is hydrogen since CO is still present in excess amounts after the end of the reaction. Now, let’s find the molar masses of each entity of the reaction medium, such as
Molar mass of CO2 = 28g
Molar mass of 2H = 4g
Molar mass of CH3OH = 28+4 = 32g
In the final step, multiply the actual mass of hydrogen with the molar mass ratio of the final product and limiting reagent such as
1.2 tons 2H2 = 32g CH3OH ÷ 4g 2H2 = 9.6 tons
Examples Of Calculating Percent Yield
Percent Yield Example 1:
Percent Yield Example 2:
A major healthcare company produces hydrogen peroxide , a chemical compound made up of hydrogen and oxygen used to clean out small cuts and scrapes. The company wants to conduct an experiment concerned with the decomposition of hydrogen peroxide to understand their processes effectiveness.
The theoretical yield of the hydrogen peroxide decomposition is 54.3. After measuring the actual yield during the reaction, it is 23.7. The companys chemist puts this information in the percent yield formula in the appropriate places.
X 100 = Percent Yield.
The chemist divides 23.7 by 54.3 to arrive at the unrefined percent yield value of 0.436.
He multiples this decimal value by 100 to get the actual percent yield.
0.436 X 100 = 43.6
The percent yield is 43.6%.
What it means: This percent yield result isnt nearly as encouraging as the one from the previous example. As stated earlier, any percent yield over 60% is generally considered to be positive productivity. 43.6% is clearly under this standard. However, this value isnt necessarily terrible.
It isnt until a percent yield value is lower than 40% that its thought of as poor and unacceptable in terms of efficiency. While the percent yield result of 43.6% is cutting it very close to being negative, it is still considered acceptable.
Recommended Reading: Glencoe Geometry Chapter 10 Answers
Calculate The Percentage Yield:
The percent yield is simply the actual yield divided by theoretical yield multiplied by 100. Actual yield is the amount of product you actually got while theoretical is the maximum possible yield. Be sure that actual and theoretical yields are both in the same units so that units cancel in the calculation.
Example:
In the reaction below we calculated a theoretical yield of 1 mol, and obtained an actual yield of 0.55 mol. What is the percent yield?
Document Actions
Percentage Yield Formula: Know Definition Examples & Uses
Percentage Yield Formula: In chemistry, the product that we obtain after a chemical reaction is known as the yield. We can measure this obtained yield in grams or moles. It is the actual yield that we get after the completion of a chemical reaction. Apart from this, based on our calculation, we calculate the expected yield of a reaction known as the theoretical yield. In this article, we have discussed the percentage yield formula in detail along with some tips to increase the annual yield.
The percent ratio of the actual yield to the theoretical yield is known as the percentage yield. This Percentage Yield is mostly lower than \ because the actual yield is mostly lower than the theoretical yield because of the incomplete reaction or loss of the product during recovery. Percentage yield can only be \ when the actual yield is equal to the theoretical yield. It is a positive value. In this article, we will learn about the percentage yield and the various factors governing it in detail.
Don’t Miss: What Is Toposheet In Geography
How To Calculate Percent Yield
With a basic understanding of what the percent yield value is demonstrating after a chemical reaction, its time to get into how to break down the somewhat unnerving formula, piece by piece.
Below are the steps for how to calculate the percent yield.
Understand the formula. To begin any math problem from basic addition to advanced trigonometry, start by taking in the formula. Although mathematical and chemical formulas are dreadful when youre unsure of what every portion means, theyre much more straightforward after analyzing each piece of the puzzle.
In the percent yield formula, two values need to be figured out and plugged in. These are the actual yield and the theoretical yield.
Find the value of the actual yield. The actual yield is the result that was achieved from this rendition of the chemical reaction. Regardless of what science says should happen, this value is what actually did happen.
Find the value of the theoretical yield. The theoretical yield is the expected result after a reaction. Chemistry explains it as the maximum anticipated result.
This could mean a lot of different things depending on what chemical medium youre working with. For example, how long it takes a chemical substance to dissipate after being heated at a particular temperature.
The theoretical yield of a particular reaction can be uncovered with research.
Example Of Bond Yield
Jaime is looking for ways to make extra money for her daughter’s college fund. She decides to purchase bonds that have a coupon of 4.5% for the price of $98.25 per bond.
Bond yield = x 100
To determine the bond’s yield, first determine the coupon amount from its percentage.
4.5% x 100 = $4.50 per bond per year
Then you can calculate the yield.
Bond yield = x 100
Bond yield = 0.0458 x 100 = 4.58% per bond
Recommended Reading: Why Do I Want To Study Geography
Lesson Explainer: Percentage Yield Chemistry
In this explainer, we will learn how to identify the limiting reagent and calculate the percentage yield of desired products based on the actual and theoretical yield.
A chemical equation can provide lots of useful information about a reaction. It can tell us about the reactants, the products formed, and the molar ratio among everything involved in the reaction. Consider the following chemical reaction between sodium hydroxide and hydrochloric acid: N
Calculate The Percent Yield
Remember the difference between percent yield and theoretical yield. Percent yield is the amount of product that is produced in a chemical reaction, as opposed to the amount that was theoretically possible. To calculate percent yield, simply divide the actual yield by the theoretical yield and multiply by 100. See the following formula:
You May Like: What Does Cyte Mean In Biology
Determine The Actual Yield Of Experiment
The actual yield is the amount of product obtained from a chemical reaction. The actual yield is obtained experimentally. The example problem states that the actual yield is 55g of azobenzene.
When not performing an experiment, the actual yield should be provided in the problem. Be sure to convert the actual yield to grams.
What Is Percent Yield
Percent yield is a formula used by chemists in the process of evaluating chemical reactions. For every chemical reaction they deal with, there is an expected result. Even with an anticipated result in mind that should happen chemically, this doesnt end up being the case the vast majority of the time.
The percent yield formula shows the chemist a percentage of how successful the reaction was in reality, compared to the maximum result they were expected.
The formula for percent yield is:
X 100 = Percent Yield
You May Like: What Does Vi Mean In Physics
Theoretical Actual And Percent Yields
The percent yield is a comparison between the actual yieldwhich is the weight of the intended product of a chemical reaction in a laboratory settingand the theoretical yieldthe measurement of pure intended isolated product, based on the chemical equation of a flawless chemical reaction, and is defined as,
When more than one reactant participates in a reaction, the yield is usually calculated based on the amount of the limiting reactant, whose amount is less than stoichiometrically equivalent to the amounts of all other reactants present. Other reagents present in amounts greater than required to react with all the limiting reagent present are considered excess. As a result, the yield should not be automatically taken as a measure for reaction efficiency.
In their 1992 publication General Chemistry, Whitten, Gailey, and Davis described the theoretical yield as the amount predicted by a stoichiometric calculation based on the number of moles of all reactants present. This calculation assumes that only one reaction occurs and that the limiting reactant reacts completely.
Calculate The Actual Yield
For calculating percent yield, first you have to calculate the actual yield. If you are performing the chemical reaction by yourself, then there will be no problem in calculating the actual yield. You have to weigh your product using a balance and note the value of the total product mass.
You can also balance your equations using online chemical equation balancer on the home page of this website.
Read Also: Navigating Through Geometry In Grades 6 8
What Is Percentage Yield
You can see from the balanced equation in the image above that 1 mole of ethene reacts with water to make 1 mole of ethanol. We can guess that if we react 28g of ethene with water, we will make 46g of ethanol. But this mass is only theoretical. In practice, the actual amount of product we get is lower than the amount we predict due to the inefficiency of the reaction process.
If you were to carry out an experiment with exactly 1 mole of ethene and excess water, the amount of product, ethanol, would be less than 1 mole. We can work out how effective a reaction is by comparing the amount of product we get in an experiment to the theoretical amount from the balanced equation. We call this percentage yield.
Percentage yield measures the effectiveness of a chemical reaction. It tells us how much of our reactants successfully transformed into a product.
Add The Values In The Percent Yield Formula
Suppose you have been asked to calculate the percent yield of a reaction whose theoretical yield is 95g while the actual yield is 92.2g. To get percent yield, insert the values in the percent yield formula, So the percent yield equation will be
Percent yield = Theoretical yield ÷ Actual yield × 100
Percent yield = 92.2g ÷ 95g × 100 = 97.0%
This is how we can find percent yield and calculate percent yield equation.
Related: A detailed article to learn about history and importance of periodic table.
Also Check: How To Learn Psychology Fast
How To Calculate Percent Yield In Chemistry In 10 Steps
To learn how to calculate percent yield in chemistry, lets review an example:
Azobenzene has the formula C6H5N and is a photoswitchable chemical. Azobenzene-based compounds are used in the textile industry as colorants and dyes.
What is the theoretical yield, percent yield, and actual yield of the following reaction if 0.10L of nitrobenzene , 0.30L of triethylene glycol yields 55g of azobenzene?
The Limiting Reagent And Excess Reagent
In a chemical reaction, you have both limiting reagents and excess reagents. The limiting reagents are the lesser in numbers and determine how much of a product will result. Therefore, limiting reagents often equals product. Excess reagents are the chemical or chemicals left over, the by-products of a reaction. The limiting reagent is important because for higher effectivity you want a balanced reaction. The ratio of limiting reagent, or limiting reactant and excess reagent, should be as close to 1:1 as possible. The more balanced the solution, the less waste results.
You May Like: Is The Chicago School Of Professional Psychology Accredited
Determine The Limiting Reagent
Calculate the molar mass of all the compounds in the chemical reaction. The molar mass is the sum of the atomic mass of each atom in a compound. For example, the molar mass of water is 18 grams: 2 grams of hydrogen plus 16 grams of oxygen.
Divide the grams of the compounds by their molar masses. This will give you the number of moles of each compound in the experiment. For example, if you initially used 36 grams of water, 36 divided by 18 grams per mole yields 2 moles of water.
Compare the moles of reactants in your experiment to the theoretical number of moles. For example, consider the chemical reaction 2F2 + 2H2O => 4HF + O2, in which “F” is fluorine, “HF” is hydrogen fluoride and “O2” is oxygen. In this case, you want equal moles of F2 and H2O. If you have 2 moles of H2O and 2.3 moles of F2, however, you have more than enough F2 to complete the reaction. Hence, H20 is the limiting reagent.
Can We Save Some Money
The world of pharmaceutical production is an expensive one. Many drugs have several steps in their synthesis and use costly chemicals. A great deal of research takes place to develop better ways to make drugs faster and more efficiently. Studying how much of a compound is produced in any given reaction is an important part of cost control.
Don’t Miss: How To Calculate Period Physics
Example : Reasons Why A Percentage Yield Is Over 100%
Which of the following explains why a reaction yield may appear to be above 1 % ?
Why Is It Important To Know Yield
It’s important to understand and monitor the yield of your investments so that you’re aware of how your securities are performing. Yield can indicate positive or negative changes in the cash you’re earning from securities. For example, a higher yield value usually means that your investment is lower risk and you’re earning a higher income. However, when yields grow too high, it can also reveal a decreasing stock price or a company that is paying higher dividends, which could be a warning sign. Lower yields can also indicate low earnings and higher risk.
Read more:
Don’t Miss: How To Do Functions In Algebra
What Does Yield Mean In Chemistry
Yield : A measure of a chemical reactions efficiency, as a ratio of moles of product to moles of reactant. Usually expressed as a percentage. % Yield = Moles of product.
What affects yield chemistry?
How do you calculate percent yield in organic chemistry?
To determine the percent yield: Divide the actual yield made in the lab by the calculated theoretical amount, and multiply by 100.
What is the yield in chemistry?
What is theoretical yield in chemistry?
Theoretical yield is the amount of product a reaction would produce if the reactants reacted completely. How many grams of Ag 2 S will form when 3.94 g of AgNO 3 and an excess of Na 2 S are reacted together?
What is the percent yield of this reaction?
The percent yield of this reaction is going to be the actual yield divided by the theoretical yield, multiplied by 100%. Its going to be 0.4 moles over 0.5 moles times 100% and we have 80%. So, the yield of this reaction is 80%.
How do you find the number of moles needed to balance reaction?
The key to solving this type of problem is to find the mole ratio between the product and the reactant. The reaction formula gives the whole number of moles needed to complete and balance the reaction. For this reaction, two moles of AgNO 3 is needed to produce one mole of Ag 2 S.
Convert All Amounts Of Reactants And Products Into Moles:
Usually reactants are measured out by volume or mass. You need to know these quantities in terms of moles to do yield calculations. The conversion of volume and mass into number of moles can be done using the density and molecular weight of the material
Mass can be converted to moles using molecular weight. Be sure to include all units in your calculations. It will help you to avoid errors. By insuring that the mass units cancel in the calculation you can be sure you have the calculation setup properly.
Example:
Consider the reaction in equation 3 above. Suppose you used 25.0 g of the reactant 3COH. To convert grams to moles use the molecularweight. So how do you know whether tomultiply or divide by the molecular weight? Answer: look at the units, grams should cancel in the calculationleaving an answer that has units of moles. This is illustrated below.
To convert volume to moles, first convert to massusing density, then convert to moles using molecular weight. Again, be sure to include all units in yourcalculations. It will help you to avoiderrors.
Example:
Againconsider the reaction in equation 3 above. Suppose you used 30.0 mL of the reactant 3COH. First convert this volume into mass usingdensity , then convert grams to moles using the molecular weight. Again, include units and set up yourcalculation so that milliliters and grams cancel in the calculation leaving ananswer that has units of moles. This isillustrated below.
You May Like: What Is Average In Math