Key Concepts And Summary
The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change . The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure . The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant . Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules .
The equations describing these laws are special cases of the ideal gas law, PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, T is its kelvin temperature, and R is the ideal gas constant.
Bodies In Thermodynamic Equilibrium
For experimental physics, hotness means that, when comparing any two given bodies in their respective separate thermodynamic equilibria, any two suitably given empirical thermometers with numerical scale readings will agree as to which is the hotter of the two given bodies, or that they have the same temperature. This does not require the two thermometers to have a linear relation between their numerical scale readings, but it does require that the relation between their numerical readings shall be strictly monotonic. A definite sense of greater hotness can be had, independently of calorimetry, of thermodynamics, and of properties of particular materials, from Wien’s displacement law of thermal radiation: the temperature of a bath of thermal radiation is proportional, by a universal constant, to the frequency of the maximum of its frequency spectrum this frequency is always positive, but can have values that tend to zero. Thermal radiation is initially defined for a cavity in thermodynamic equilibrium. These physical facts justify a mathematical statement that hotness exists on an ordered one-dimensional manifold. This is a fundamental character of temperature and thermometers for bodies in their own thermodynamic equilibrium.
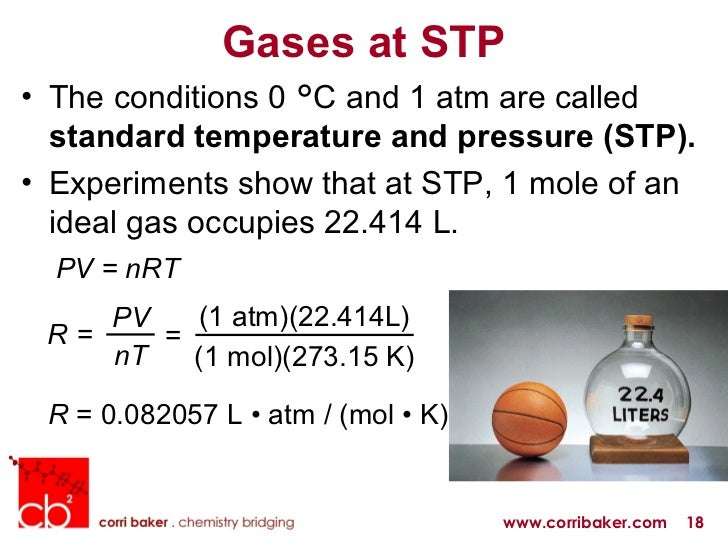
Conditions For Standard Temperature And Pressure
The standard temperature and pressure are the conditions used in thermodynamics to figure out the reference points of various gases. These are also used to specify the vapor volume. The gas volumes can be converted to the number of moles which makes it easier to find the reference points. The volume of a gas varies with temperature and pressure hence it makes it impossible to measure the molar quantity. When the gas volume is fixed to its temperature and pressure, then the standard volume is directly proportional to the molar quantity.
With the help of standard temperature and pressure, the compositions of gases can also be calculated. The value of temperature and pressure depends on the organization which defines them. The standard pressure is equal to the atmospheric pressure and the temperature is close to room temperature. NTP is a term that is referred to as normal temperature and pressure. The NTP uses 20°C as the standard temperature whereas the standard temperature and pressure use 0°C as the standard temperature. The NTP was used by the National Institute of Standards and Technology. The calculation of volume at NTP is given as
You May Like: What Is The Definition Of Mantle In Geography
Breathing And Boyles Law
What do you do about 20 times per minute for your whole life, without break, and often without even being aware of it? The answer, of course, is respiration, or breathing. How does it work? It turns out that the gas laws apply here. Your lungs take in gas that your body needs and get rid of waste gas . Lungs are made of spongy, stretchy tissue that expands and contracts while you breathe. When you inhale, your diaphragm and intercostal muscles contract, expanding your chest cavity and making your lung volume larger. The increase in volume leads to a decrease in pressure . This causes air to flow into the lungs . When you exhale, the process reverses: Your diaphragm and rib muscles relax, your chest cavity contracts, and your lung volume decreases, causing the pressure to increase , and air flows out of the lungs . You then breathe in and out again, and again, repeating this Boyles law cycle for the rest of your life .
Figure 7.
Bodies In A Steady State But Not In Thermodynamic Equilibrium
While for bodies in their own thermodynamic equilibrium states, the notion of temperature requires that all empirical thermometers must agree as to which of two bodies is the hotter or that they are at the same temperature, this requirement is not safe for bodies that are in steady states though not in thermodynamic equilibrium. It can then well be that different empirical thermometers disagree about which is hotter, and if this is so, then at least one of the bodies does not have a well-defined absolute thermodynamic temperature. Nevertheless, anyone has given body and any one suitable empirical thermometer can still support notions of empirical, non-absolute, hotness, and temperature, for a suitable range of processes. This is a matter for study in non-equilibrium thermodynamics.
Don’t Miss: Algebra 2 Sequences And Series
What Is Standard Temperature And Pressure
Some textbooks define it differently than others, but the newest IUPAC standard temperature and pressure are:
Some older textbooks might say:
The difference between the two pressures are subtle but significant:
#”1 bar” = 1.00000xx10^5#
This leads to a difference of about #”0.3 L”# of an ideal gas at STP when you calculate it using the Ideal Gas Law: #~”22.7 L”# #~”22.4 L”# for the latter.
Ever had your university lab notebook “torn apart” by a lab TA for “not enough information”? Yeah, it’s primarily because science tends to rely on consistency and reproducibility to prove that something is credible.
If someone can’t read your lab notebook and then reproduce your lab experiment without your input and correction, you haven’t provided enough information to replicate that experiment precisely.
IUPAC has defined such standards so that people have consistent atmospheric conditions to use for comparisons of data from different experimental trials for the same type of experiment. That improves the accuracy to which an experiment can be reproduced.
SIDENOTE: This is not be confused with the temperature and pressure at which #DeltaH_f^@#
Overview Of Standard Temperature And Pressure
STP is an abbreviation for standard temperature and pressure. It is a set of conditions for temperature and pressure measurements for physical and chemical reactions. The standard temperature is zero º Celsius, which corresponds to 32 °F or 273.15 K. The standard pressure is 1 bar or 100.000 kPa. The molar volume of a gas is important for identifying th conditions of temperature and pressure when stating the molar volume of a gas. The molar volume of gas can be calculated by the universal gas law. The temperature and pressure are state functions. Temperature changes according to the location and season, whereas pressure depends on the weather conditions and the height above the sea level. STP is not a standard state condition. A standard state is used in thermodynamics calculations.
You May Like: What Is The Electron Geometry Of So3
Definitions Of Standard Temperature And Pressure
The term STP means different things to different people and can cause problems in the presentation of adsorption data, because the most common units for the ordinates of such plots are standard volumes per unit mass of adsorbent.
The International Union of Pure and Applied Chemistry used to define STP as 0 C and 1 atm . This definition is now obsolete. The preferred definition, since 1982, is 273.15 K and 1 bar . The National Institute of Standards and Technology, on the other hand, defines STP as 1 atm and 20 C . In practical applications of adsorption equipment, STP often refers to the pressure of the room and the temperature of the adsorption manifold, which is often kept above room temperature to prevent changes in room temperature from changing the apparent manifold or dead volumes . Room temperature, at least in the summer months in many places, is usually close to 25 C, which is a de facto standard in gas flow controllers.
For the sake of comparison, we recommend specifying the definitions of STP being used if there is any concern that they will affect the results. The difference between using a standard temperature of 0 C and 25 C will, in the authors’ experience, produce errors in the volume adsorbed that are less than the error in the measurements themselves. In commercial equipment, the volume adsorbed is usually reported by defining STP as 0 C and 760 Torr .
Alain Tressaud, in, 2019
What Are The Conditions For Stp In Chemistry
4.9/5temperaturetemperature
In respect to this, is STP 25 or 0?
Both STP and standard state conditions are commonly used for scientific calculations. STP stands for Standard Temperature and Pressure. It is defined to be 273 K and 1 atm pressure . Temperature is not specified, although most tables compile data at 25 degrees C .
Subsequently, question is, what does STP stand for? Standard Temperature and Pressure
In this way, what is the standard value for pressure at STP condition?
Standard Temperature and Pressure. Standard temperature is equal to 0 °C, which is 273.15 K. Standard Pressure is 1 Atm, 101.3kPa or 760 mmHg or torr. STP is the standardconditions often used for measuring gas density and volume.
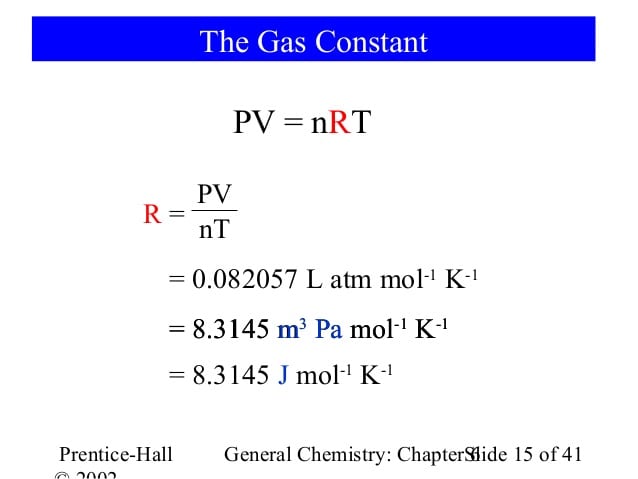
What is PV nRT called?
PV = nRT: The Ideal Gas Law. Fifteen ExamplesEach unit occurs three times and the cube root yields L-atm / mol-K, the correct units for R when used in a gas law context. Consequently, we have: PV / nT = R. or, more commonly: PV = nRT. R is the gas constant.
Also Check: Chapter 9 Review Algebra 2
What Is Standard Temperature And Pressure In Chemistry
Answer:
Explanation:
- Standard temperature and pressure, abbreviated STP, refers to nominal conditions in the atmosphere at sea level. This value is important to physicists, chemists, engineers, and pilots and navigators.
- Standard temperature is defined as zero degrees Celsius , which translates to 32 degrees Fahrenheit or 273.15 degrees kelvin . This is essentially the freezing point of pure water at sea level, in air at standard pressure.
- Standard pressure supports 760 millimeters in a mercurial barometer . This is about 29.9 inches of mercury, and represents approximately 14.7 pounds per inch . Imagine a column of air measuring one inch square, extending straight up into space beyond the atmosphere. The air in such a column would weigh about 14.7 pounds.
- The density of air at STP is approximately 1.29 kilogram per meter cubed . This fact comes as a surprise to many people a cubic meter of air weighs nearly three pounds!
- See also: SI , specific gravity.
Chemistry End Of Chapter Exercises
the appropriate graph
Boyles law
Read Also: Electron Pair Geometry Of H2o
Why Do We Need Satp
SATP is brought by the International Union of Pure and Applied Chemistry . It is used in Chemistry as a reference standard condition. The standard ambient temperature in SATP is 25 . This temperature is more practical and convenient compared to 0 of STP . It would be easier for chemists all over the world to take SATP conditions as a reference point compared to STP conditions.
Why Do We Need Standards
Chemists require STP definitions because the behavior of a substance varies greatly depending on the temperature and pressure. STP definitions give chemists a common reference point to describe how a gas behaves under normal conditions. Scientists use standards like STP definitions for two purposes, to define certain quantitative metrics and to allow for consistent and repeatable experiments.
Imagine someone tells you the molar volume of methane is 22.4 liters . The molar volume of a substance is just a measure of how much space one mole of that substance takes up. On its own, this value is not very informative. It is known that the volume of a gas varies greatly with respect to pressure and temperature, so gas could have multiple molar volumes, depending on the exact temperature and pressure. One needs to specify a temperature and pressure to make a molar volume measurement of 22.4 L a more meaningful quantity. Scientists agree upon a predefined temperature and pressure to report quantitative properties of gases. As it just so happens, one mole of any gas at STP has a volume of 22.4 L. Quantitative measurements of gas, like volume, volumetric flow, and compressibility, all must be defined with respect to some defined pressure and temperature.
The true method of knowledge is experiment. William Blake
Also Check: What Does Cohesion Mean In Biology
Correct Use Of The Term Stp
Even though STP is defined, you can see the precise definition depends on the committee that set the standard! Therefore, rather than citing a measurement as performed at STP or standard conditions, it’s always best to explicitly state the temperature and pressure reference conditions. This avoids confusion. In addition, it is important to state the temperature and pressure for the molar volume of a gas, rather than citing STP as the conditions. When calculated molar volume, one should state whether the calculation used the ideal gas constant R or the specific gas constant Rs. The two constants are related where Rs = R / m, where m is the molecular mass of a gas.
Although STP is most commonly applied to gases, many scientists try to perform experiments at STP to SATP to make it easier to replicate them without introducing variables. It’s good lab practice to always state the temperature and pressure or to at least record them in case they turn out to be important.
Volume And Pressure: Boyles Law
If we partially fill an airtight syringe with air, the syringe contains a specific amount of air at constant temperature, say 25 °C. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases if we pull out the plunger, the volume increases and the pressure decreases. This example of the effect of volume on the pressure of a given amount of a confined gas is true in general. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume increases by a certain factor, the pressure decreases by the same factor, and vice versa. Volume-pressure data for an air sample at room temperature are graphed in Figure 5.
Figure 5.PVPV
Unlike the PT and VT relationships, pressure and volume are not directly proportional to each other. Instead, P and V exhibit inverse proportionality: Increasing the pressure results in a decrease of the volume of the gas. Mathematically this can be written:
Figure 6.PV
The relationship between the volume and pressure of a given amount of gas at constant temperature was first published by the English natural philosopher Robert Boyle over 300 years ago. It is summarized in the statement now known as Boyles law: The volume of a given amount of gas held at constant temperature is inversely proportional to the pressure under which it is measured.
Don’t Miss: What Is C5 In Chemistry
Physical Chemical Characteristics Of Fluorine
Table 1. Physicalchemical data of fluorine
| Melting point |
| O2+ | N2+ |
On March 23, 1962, when platinum hexafluoride, a red gas, was allowed to mix with a large molar excess of xenon , the immediately formed product was a yellow solid with the composition Xe+ , the first synthesized compound of a noble gas.99
Fig. 27. Synthesis of the first compound of noble gases by N. Bartlett: Xe and PtF6 gases prior to reaction resulting XePtF6.
This discovery was acclaimed101 as one of the ten most beautiful experiments in chemistry, because102 it opened the way for the noble gas chemistry and high oxidation species chemistry, as illustrated by the various species isolated in Neil’s Berkeley Group: first example of Os + VII: OsOF5 NOWF7, 2WF8, NOReF7 Isolation of ONF3: 2NiF6 ONF + ONF3 + NiF2 synthesis and oxidizing properties of ReOF5 and OsOF5 salts of quinquevalent gold: M+ AuF6, NO+ AuF6, Xe2F11+ AuF6, XeF5+ AuF6, etc. Quoting P. Ball, we can say that Neil Bartlett’s experiment tells us that in chemistry, the wonders never cease.103
Now, fluorine gas is produced following the electrochemical process, as shown, for instance at Fig. 28 for the F2 synthesis facilities of Advance Research Chemicals, Inc. . An important technical point is that hooded areas should be specially equipped for fluoride manufacturing. All work should be done in hoods, 40,000 cfm fresh air being pulled in to avoid depression in the building.
Fig. 28. Fluorination facilities at ARC, Inc.