Solution For Problem 53p Chapter 3
Chemistry | 11th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
Chemistry | 11th Edition
The molar mass of caffeine is 194.19 g. Is the molecular formula of caffeine \ or \?
Here, we are going to determine the molecular formula of caffeine.
We know that,
The molar mass of C = 12.01 g
The molar mass of H=1.008 g
The molar mass of N= 14.00 g
The molar mass of O=16.00 g
ISBN: 9780073402680
The full step-by-step solution to problem: 53P from chapter: 3 was answered by , our top Chemistry solution expert on 11/08/17, 03:59AM. This full solution covers the following key subjects: Caffeine, mass, formula, Molar, Molecular. This expansive textbook survival guide covers 25 chapters, and 3257 solutions. This textbook survival guide was created for the textbook: Chemistry, edition: 11. Chemistry was written by and is associated to the ISBN: 9780073402680. The answer to ?The molar mass of caffeine is 194.19 g. Is the molecular formula of caffeine \ or \? is broken down into a number of easy to follow steps, and 17 words. Since the solution to 53P from 3 chapter was answered, more than 3245 students have viewed the full step-by-step answer.
Other solutions
Redefinition Of Si Base Units
In 2011, the 24th meeting of the General Conference on Weights and Measures agreed to a plan for a possible revision of the SI base unit definitions at an undetermined date.
On 16 November 2018, after a meeting of scientists from more than 60 countries at the CGPM in Versailles, France, all SI base units were defined in terms of physical constants. This meant that each SI unit, including the mole, would not be defined in terms of any physical objects but rather they would be defined by constants that are, in their nature, exact.
Such changes officially came into effect on 20 May 2019. Following such changes, “one mole” of a substance was redefined as containing “exactly 6.02214076×1023 elementary entities” of that substance.
What Is Molar Mass
Relative molar mass is defined as the smallest mass unit of a compound with one twelfth of the mass of one carbon 12 atom.
In a substance, the amount of entities present for e.g. atoms, molecules, ions, is defined as a mole. A mole of any substance is\ molecules. Just as we take a standard value to calculate different things e.g. 1 dozen =12 items similarly we use the mole to calculate the size of the smallest entities quantitatively.
Also Check: Is Physics Harder Than Chemistry
Example : To Determine Molar Mass Of Sodium Chloride Solution
Sodium chloride a common salt. It is white and odourless powder. Consider a solution of 50 % NaCl. 100 g of the solution consists of 50 g of NaCl and 50 g of H2O.
The molar mass of water is 2 × 1.008 + 15.999 = 18.015 g mol1 and of sodium chloride is 22.99 + 35.45 = 58.44 g mol1.
The molar of the solution is calculated as follows:
Thus, the molar mass of 50 % sodium chloride solution is 28 g mol1.
Nature Of The Particles
The mole is essentially a count of particles. Usually the particles counted are chemically identical entities, individually distinct. For example, a solution may contain a certain number of dissolved molecules that are more or less independent of each other. However, in a solid the constituent particles are fixed and bound in a lattice arrangement, yet they may be separable without losing their chemical identity. Thus the solid is composed of a certain number of moles of such particles. In yet other cases, such as diamond, where the entire crystal is essentially a single molecule, the mole is still used to express the number of atoms bound together, rather than a count of multiple molecules. Thus, common chemical conventions apply to the definition of the constituent particles of a substance, in other cases exact definitions may be specified.The mass of 1 mole of a substance is equal to its relative atomic or molecular mass in grams.
Read Also: What Is The Geography Of Paraguay
What Is The Percentage Of Composition
We can find the percentage composition of a substance by dividing the mass of that substance to the total mass of the substance. Suppose we have to find out the percentage composition of hydrogen in butane) then it will be:
The total mass of one mole of butane =58.123
Mass of hydrogen in one mole of butane = 10.0794
Therefore, mass percent of hydrogen in butane=\=\
These concepts play a very important role in studying the behaviour of matter under different conditions.
How Does The Molar Mass Calculator Work
We take the formula you provide and unpack it into the component elements. Then wecompare each atom against a table of the standard atomic weights for that element. We present the results in a table at the bottom ofthe molar mass calculator – it will show the count of atoms, the atomic weight of each element, and the molecular weight for the molecule.
We don’t have brackets implemented , so you will need to unpack any bracketed expressions. They don’t affect the weight anyhow.Simply take each element and multiple it by the number of times the bracketed structure occurs. For example:3PCCO => C18H15PCCO
You May Like: Geometry Segment Addition Postulate Worksheet
How Do You Calculate The Molar Mass Of A Chemical
To calculate molar mass, you will need to know at least two things. These are:
- names of elements and their atomic masses
- chemical formula of the chemical substance
For names of elements and their atomic masses, we can easily find that information from the periodic table. For chemical formula of chemical substance, we are usually given that information in the question or asked to deduce it from given information. Now, lets apply these principles to work the following examples:
Example
Calculate the molar mass of sodium
Solution
From the question, we have only one atom of Na. This means, we simply locate Na on the periodic table and read its atomic mass. If you read it correctly and rounded it to two decimal places, you should get 22.99. Now, to get its molar mass, we must attach the unit grams per mol to its atomic mass. Thus, molar mass of sodium is 22.99 g/mol. Grams per mol is another way of saying that for every 1 mole of Na, there are 22.99 g of sodium atoms in it. If you are confused about this, click on this link to review the mole concept.
Example
Calculate the molar mass of CuSO4.5H2O sulfate pentahydrate)
Solution
How to calculate molar mass of compound with water in it
Converting Mass To Number Of Moles
How many moles of NaOH are present in 90 g of NaOH?
Since the molar mass of NaOH is 40 g/mol, we can divide the 90 g of NaOH by the molar mass to find the moles of NaOH. This the same as multiplying by the reciprocal of 40 g/mol.
If the equation is arranged correctly, the mass units cancel out and leave moles as the unit.
90\text\space \text \times \frac}} = 2.25 \space \text
There are 2.25 moles of NaOH in 90g of NaOH.
Read Also: Broward County Public Schools Algebra 1 Countdown Answers
Iifeffect Of Molar Mass On Viscosity
Molar mass has a significant effect on the rheological properties of polymer melts and hence on their processing performance. At low molar mass, i.e., below some critical molar mass , for flexible chain polymers 0 depends on Mw, while above Mc, 0 depends on Mw to the 3.4 to 3.6 power for most flexible linear polymer chains:
The 3.4 power dependence has been observed experimentally and predicted theoretically. Furthermore, the primary normal stress difference coefficient in the limit as the shear rate goes to zero, 1,0, is observed to be proportional to Mw raised to the 7.0 power, i.e.:
For branched polymers the dependence of 0 on Mw can be to higher or lower powers than 3.4 to 3.6 depending on the molecular weight between branch points. For rodlike molecules there is some evidence that the following relations hold:
In addition to the dependence of the magnitude of 0 and 1,0 on Mw, the onset of shear-thinning behavior is affected by M. As M increases, the onset of shear thinning moves to lower shear rates. An increase in the breadth of the molar mass distribution will also cause shear thinning to occur at lower shear rates.
FIGURE 13. Melt flow index device.
El-Zeiny M. Ebeid, Mohamed B. Zakaria, in, 2021
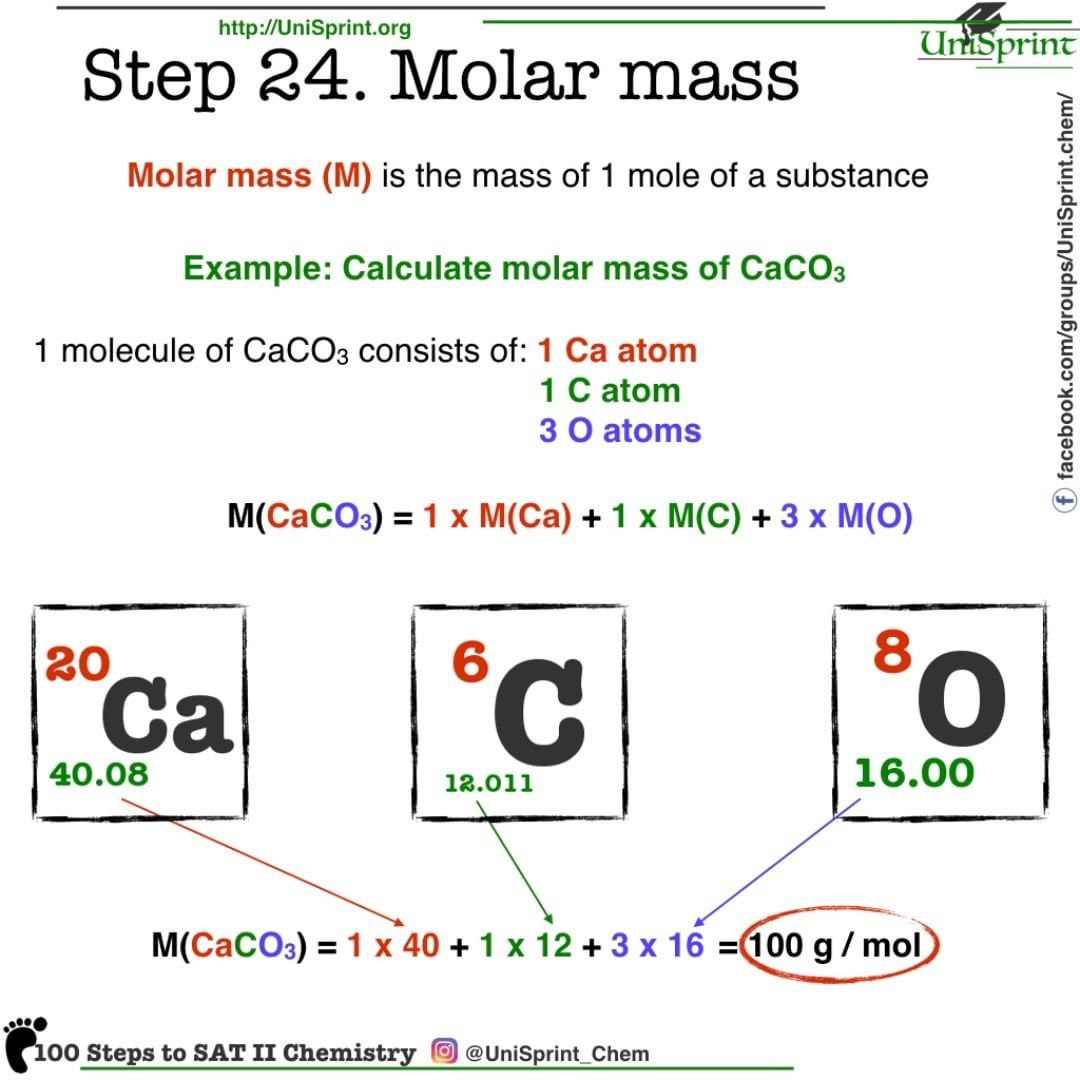
Molar Masses Of Compounds
The molar mass of a compound is given by the sum of the standard atomic weights of the atoms which form the compound multiplied by the molar mass constant, Mu:
- M = × 1 g/mol = 58.443 g/mol
- M = × 1 g/mol = 486.424 g/mol
An average molar mass may be defined for mixtures of compounds. This is particularly important in polymer science, where different polymer molecules may contain different numbers of monomer units .
Recommended Reading: Define Movement In Geography
How Are Seborrheic Keratoses Treated
Seborrheic keratoses are harmless and are not contagious. Therefore, they don’t need to be treated.
If you decide to have seborrheic keratoses removed because you don’t like the way they look, or because they are chronically irritated by clothing, methods for removing them include cutting them off, cryosurgery, and electrosurgery.
S To Find Molar Mass For Compounds
Compounds are those substances that are made up of one or more elements. Examples of some common compounds include glucose, salt, acetic acid, and sodium bicarbonate.
Sodium chloride compound is made up of two elements i.e., sodium and chloride. We will use sodium chloride as one of the examples to calculate the molar mass for the compounds
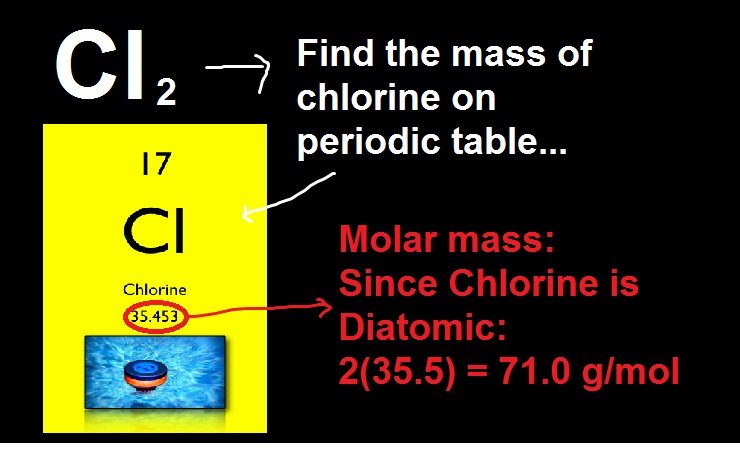
Step 1: Find the atomic mass of individual element in the periodic table
We have to first find the atomic mass for each element. The element sodium has the atomic mass of 22.98976g/mol. Chlorine has an atomic mass of 35.453 g/mol.
Step 2: Count atoms for each element
As there are no subscripts for compound sodium chloride, it means it has only one sodium and one chlorine atom for the compound.
Step 3: find the molar mass
Now, we are able to find the molar mass as we know the number of atoms for each element. Here we calculate first the mass of the sodium atoms that is 22.98976 g/mol. we will repeat the same for mass of chlorine atoms that is 35.453 g/mol, now we have to add these two masses together to find the total mass of molecules of sodium chloride. The total sodium chloride molecules are 58.44276 g/mol which we can round up to 58.44 g/mol.
Na = 1 × = 22.98976 g/mol
Cl = 1 × = 35.453 g/mol
Molar mass = 22.98976 + 35.453 g/mol
Molar mass = 58.44276 or 58.44 g/mol
You May Like: Fsa Algebra 1 Eoc Practice Test Answers
Bookmarking Save And Share Results
The tool is designed so you can flip between different parts of a problem set. We recommend you bookmark it so you can refer back to it.You can also by hitting calculate and copying the URL for this page. When your study partneropens up the URL, they will see your calculations. It’s easy share & save results via email.
You also have the option of saving links to the calculations in your research notes files, so you can quickly re-open or check them later.Again – hit calculate first so the URL is updated with your most recent changes. Then copy and save the url.
What Is Molar Mass Dependent On
4.1/5molar massmassmolar massdepend
symbol M
Additionally, does molar mass change with temperature? In other words, as the temperature of a sample of gas is increased, the molecules speed up and the root mean square molecular speed increases as a result. And conversely, lighter the molar mass of the gas molecules the faster the gas molecules move.
is relative molecular mass the same as molar mass?
The molecular mass and relative molecular mass are distinct from but related to the molar mass. The molar mass is defined as the mass of a given substance divided by the amount of a substance and is expressed in g/mol.
What is Mole and molar mass?
A mole is 6.021023 molecules of a substance. The molar mass is the amount of mass that 1 mole of that substance possesses.
Read Also: What Is The Molecular Geometry Of Ccl4
Solved Examples Of Molar Mass
1. When you have 1.25 grams of a molecule with a molecular weight of 134.1 g/mol, find out how many moles of that molecule you have?
Solution: 1.25 g × 1 mole / 134.1 g = 0.0093 grams.
2. Calculate the mass of 6.022 × 1023 molecules of NH4Cl?
Solution: Molar mass in grams of NH4Cl = 14 + 4 +35.5 = 53.5 g
No. of moles of NH4Cl = 6.022 × 1023 / 6.022 × 1023 = 1 mole.
Now, mass of NH4Cl = number of moles × molar mass
= 1 × 53.5 g
= 53.5 g
3. Calculate the number of methane molecules and the number of carbon and hydrogen atoms in 25 g of methane?
Solution: Molar mass of methane = 16
Number of methane molecules = 25/16 × 6.022 × 1023
= 9.411 × 1023
Number of carbon molecules = 1 × 9.411 × 1023
= 9.411 × 1023
Number of hydrogen molecules = 4 × 9.411 × 1023
= 3.74 × 1023
Effect Of Molecular Variables And Morphology On Fracture Behavior
The molar mass is well known to play a key role in the ability of thermoplastic polymers to initiate and sustain plastic deformation . A low molar mass has three immediate consequences. First, there is a correspondingly high concentration of chain ends, which act as structural defects. Second, if the chains are short relative to the interlamellar spacing, the probability of their being incorporated in two different lamellae simultaneously, thus forming a tie-molecule, also becomes small. For a linear PE of mass 100 kg mol-1, the average chain end-to-end distance in the melt, which is substantially maintained in the solid, is of the same order of magnitude as the lamellar spacing, i.e., typically 15 to 30 nm depending on the lamellar thickness and the degree of crystallinity . Moreover, even where effective molecular connections between the lamellae exist, they may become highly unstable with respect to chain disentanglement when the molar mass is low, particularly at low deformation rates . Under these conditions, the tensile strain at break, eb, is generally no more than a few percent.
Figure 5. Toughness of different polymers as a function of ?e1/2.
Don’t Miss: Who Are The Biological Parents Of Prince Paris And Blanket
Molar Mass Versus Molecular Mass
Molar mass is sometimes confused with the related but distinct molecular mass. This is largely due to that when the molar mass and molecular mass are expressed in g/mol and u respectively they will almost always have similar but not identical numerical values. The molar mass is generally computed from isotopically weighted averages, whereas the molecular mass is the mass of a single molecule consisting of well-defined isotopes. The isotopically weighted averages used to compute molar masses are those found in most versions of the periodic table and are numbers recommended by IUPAC. They represent the most likely weights of substances found in the laboratory. The averaging takes into account the natural abundance of, usually heavier, isotopes as well as the variation in their natural abundance in different places on earth. Additionally the confidence, or number of significant figures after the decimal, is different. The significant figures in the standard atomic weights and thus the computed molar masses are often limited by the natural variations in the isotopic distributions and not necessarily by our ability to measure accurately. The confidence in the isotopic masses and resulting molecular masses are only limited by the accuracy of measurement of the invariable isotopic masses.
Chapter 1: The Mole And Molar Mass
- To calculate the molecular mass of a covalent compound.
- To calculate the formula mass of an ionic compound.
- To calculate the number of atoms, molecules or formula units in a sample.
Chemistry is the study of how atoms and molecules interact with each other which occurs on the atomic scale. Chemists need a way of simply determining how many molecules they have in a beaker. The mole concept, which we will introduce here, bridges that gap by relating the mass of a single atom or molecule in amu to the mass of a collection of a large number of such molecules in grams.
Also Check: How To Do Conversions In Chemistry
Can Lentigines Be Prevented
The best way to prevent lentigines is to stay out of the sun as much as possible, especially between the hours of 10 a.m. and 2 p.m. Use a broad-spectrum sunscreen with an SPF of 30 or higher when outdoors, and wear protective clothing, such as long-sleeved shirts, pants, and a wide-brimmed hat. Avoid using tanning beds.
How Is A Mole Calculated
If you want to know how many moles of a material you have, divide the mass of the material by its molar mass. The molar mass of a substance is the mass in grams of one mole of that substance. This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units . For example, silver has an atomic weight of 107.8682 amu, so one mole of silver has a mass of 107.8682 grams.
mole, also spelled mol, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
The mole designates an extremely large number of units, 6.02214076 × 1023. The General Conference on Weights and Measures defined the mole as this number for the International System of Units effective from May 20, 2019. The mole was previously defined as the number of atoms determined experimentally to be found in 12 grams of carbon-12. The number of units in a mole also bears the name Avogadros number, or Avogadros constant, in honour of the Italian physicist Amedeo Avogadro . Avogadro proposed that equal volumes of gases under the same conditions contain the same number of molecules, a hypothesis that proved useful in determining atomic and molecular weights and which led to the concept of the mole.
Recommended Reading: What Is The Molecular Geometry Of Ccl4