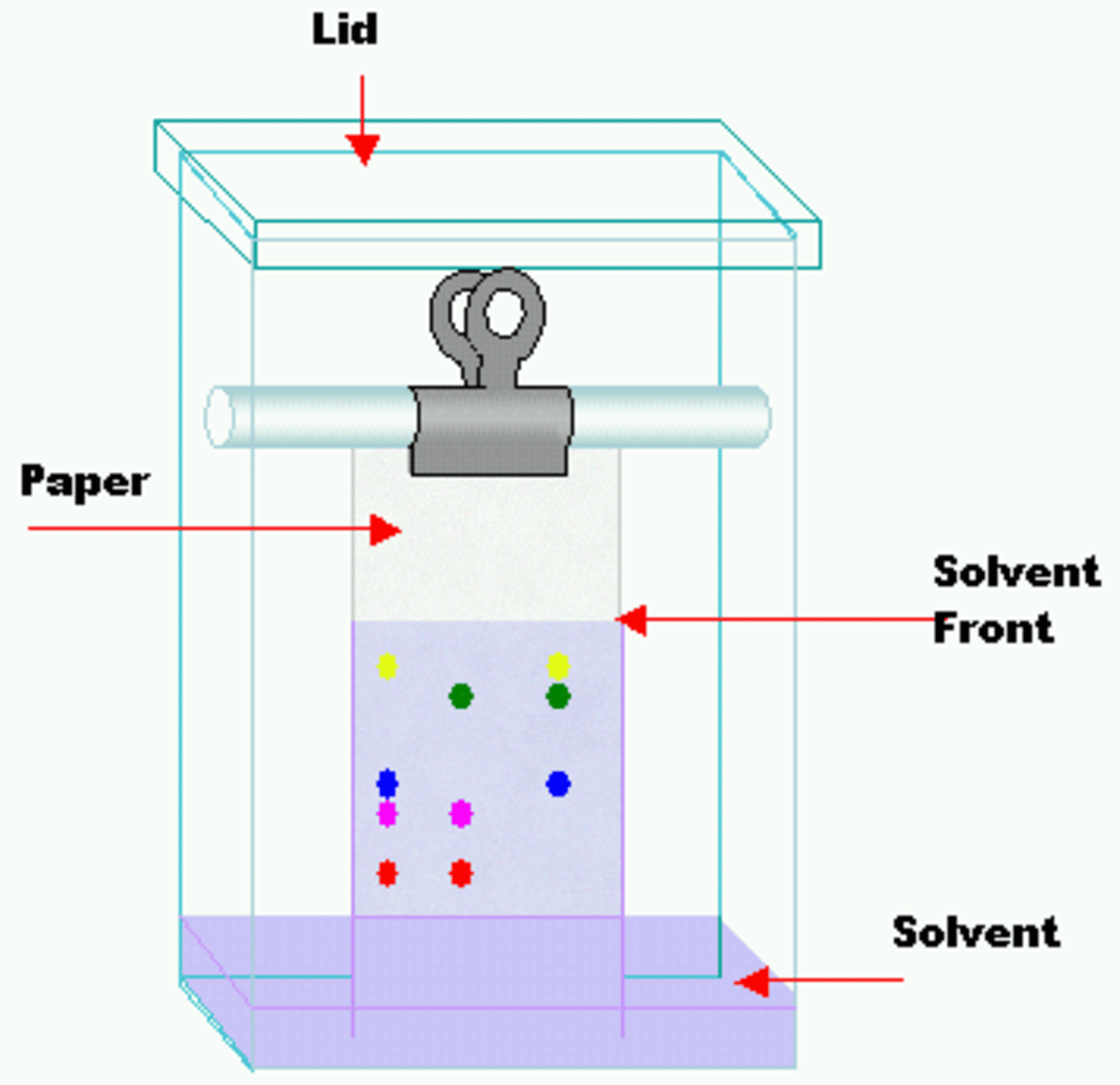
How Does Hplc Work
In column chromatography, a solvent drips through a column filled with an adsorbent under gravity. HPLC is a highly improved form of column chromatography. A pump forces a solvent through a column under high pressures of up to 400 atmospheres. The column packing material or adsorbent or stationary phase is typically a granular material of solid particles such as silica or polymers.
The pressure makes the technique much faster compared to column chromatography. This allows using much smaller particles for the column packing material. The smaller particles have a much greater surface area for interactions between the stationary phase and the molecules flowing past it. This results in a much better separation of the components of the mixture.
The pressurized liquid is typically a mixture of solvents such as water, acetonitrile and/or methanol and is referred to as the mobile phase.
The components of a mixture are separated from each other due to their different degrees of interaction with the absorbent particles. This causes different elution rates for the different components and leads to the separation of the components as they flow out the column. Compared to column chromatography, HPLC is highly automated and extremely sensitive.
The two most common variants are normal-phase and reversed-phase HPLC.
Factors Affecting Hplc Separations
Many factors, including mobile phase composition, stationary phase chemistry, and temperature influence HPLC separations. Successful separation only occurs if the analytes have differing affinities for the stationary phase, so selecting the appropriate stationary phase for your compounds is crucial. The main factors influencing the overall separation process are:
- Physiochemical properties of the analyte, such as size, charge, polarity, and volatility
- Physiochemical properties of the stationary phase, such as polarity, charge, and viscosity
- Physiochemical properties of the mobile phase used and interaction with the analyte and stationary phases
All HPLC separations are carried out in one of two modes, isocratic or gradient.
Isocratic methods separate by using a consistent eluent composition during analysis, like 100% acetonitrile or a 50:50 mixture of acetonitrile to water.
On the other hand, gradient methods include a change in the mobile phase composition across a separation. These methods often employ two solvents, called A and B. The run will begin with a certain percentage of A to B, like 60% water to 40% acetonitrile, for instance, followed by a percentage change throughout a separation.
Gradient separations usually provide superior performance over isocratic modes but are more complex and require advanced pump hardware.
How Does Pure Water Affect Hplc
HPLC is incredibly dependent upon water purity. Using an impure water source to prepare eluents, blanks, samples and standards could introduce contamination into the experiment, degrading the chromatographic performance by impacting resolution, integration and baselines. As water is the reagent used in the largest volume in HPLC, it is vital that the water chosen is of the correct purity required for the sensitivity of the application.
Which water type should you use?For HPLC experiments where the applications have a general sensitivity, we recommend Type II+ water. Where the sensitivity of the application is high, Ultrapure Type I+ water should be used as it has a resistivity of more than 18 M.cm, a TOC value of less that 2ppb, less than 1 CFU/mL of bacteria and less than 0.03 endotoxins.
Also Check: What Is Basic Research In Chemistry
To Analyze All Of The Compounds In A Complex Mixture
This might be useful in microbiology and environmental monitoring. Lets say that a new mold has been found growing in the corners of old homes. This could potentially pose a health threat, as certain molds are known to produce toxic metabolites. You could use HPLC to investigate the compounds that this species produces to create a metabolite profile for this species. This will help you to determine whether or not it is a threat.
In this situation, HPLC can probably aid in identifying some of the compounds through comparisons with the metabolite profiles of other well-known molds. However, for a meaningful comparison, all samples should be tested under exactly the same conditions .
Advances In Stationary Phase Technologies
Silica-based columns have remained the backbone of stationary phases to this day. However, columns with monolithic phases, zircon base phases and those based on core shell-based technologies have contributed to greater speed of analysis, wider temperature and pressure range of operation, and studies over extended pH range of media.
Don’t Miss: One To One Linear Algebra
How Do You Understand The Output
You will usually see the results of an HPLC run in the form of a chromatogram. This shows horizontal peaks which represent the compounds flushed out of the column and their Rf. Most modern HPLC machines come with a diode array detector .
This will let you look at the chromatogram in wavelengths from 190nm to 900nm. If you know what compounds were flushed out, you can single out 1 or a few wavelengths. For example, cocaine shows up at 254nm.
Water Purification Systems Supplied By Elga Labwater
If youre in need of pure water for your HPLC applications, have a look through our Water Purification Systems, designed to provide you with an efficient supply of the water grade you need, whether it be Ultrapure or Type III. Whether you want to request a demo or ask a question, get in contact today.
ELGAs expertise and long-established reputation ensure that its experienced team can help customers to determine the particular water purity requirements for their applications. The Company offers a number of water purification systems that have been proved to meet the requirements for HPLC. For example, the bench-top PURELAB Chorus 1 Analytical Research point-of-use system consistently delivers ultrapure water of 18.2 M.cm and TOC less than 2ppb suitable for all these applications.
You May Like: How Can Biology Be Studied At Different Scales
What Are The Different Uses Of Hplc
HPLC is a commonly used and extremely powerful chromatographic technique, with applications in areas such as pharmaceutical, bioanalytical, food and beverage, clinical, forensic, environmental and drug development laboratories.
For example, in a medical setting HPLC can be used to determine the contents and concentrations of substances in biological materials. This could include drug analysis of urine or detection of vitamin levels in blood serum.
Different Combinations Of These Parts Are:
· Pumps
· Detectors
· Injectors yield an infinite number of configurations based on the application
You need to have a good understanding of the parts of your HPLC system and the HPLC principles to generate data of the highest reliability. A conceptual understanding of the function of each component will add to your comfort level with your HPLC system, and you will ensure long term usage with high reliance on output data.
Don’t Miss: What Is An Inertial Frame Of Reference In Physics
The Support You Need To Optimize Operations
Avantor Services provides a wide range of specialized services and digital solutions to help you solve complex challenges.
Weve built our reputation on consistent, comprehensive mastery of day-to-day operations, allowing lab, clinical, and production environments to focus their high-value resources on core scientific priorities.
As our customers needs have evolved, so have our capabilities. We have become experts in scientific operations, improving performance with sophisticated solutions and providing guidance on best practices.
You can select and customize services for peak efficiency, quality, and accelerated innovation.
For more information, call 1.888.793.2300 or email us at .
VWR enables the advancement of science by providing high-quality chemicals and services, customized to your product or manufacturing needs.
We use operational excellence to deliver solutions that enable research, testing, production, and commercialization across the globe.
Our Core Capabilities Include:
- Custom Liquid Dosing and Packaging
- Custom Powder Manufacturing
The Apparatus Of Hplc
The âBasic Overview of the HPLC process” and its mechanisms have now been covered. Going into more detail, HPLC consists of a variety of components, including a solvent delivery pump, a degassing unit, a sample injector, a column oven, a detector, and a data processor. Fig.2 shows the HPLC flow diagram and the role of each component.
Fig.2ãHPLC Flow Diagram
As for HPLC, the pump delivers the mobile phase at a controlled flow rate. Air can easily dissolve in the mobile phase under the standard atmospheric pressure in which we live in. If the mobile phase contains air bubbles and enters the delivery pump, troubles such as flow rate fluctuations and baseline noise/drift may occur. The degassing unit helps prevent this issue by removing air bubbles in the mobile phase. After the dissolved air has been removed, the mobile phase is delivered to the column. The sample injector then introduces a standard solution or sample solution into the mobile phase . Temperature fluctuations can affect the separation of compounds in the column. The column is placed in a column oven to keep the temperature constant. Compounds eluted from the column are detected by a detector which is placed downstream of the column. A workstation processes the signal from the detector to obtain a chromatogram to identify and quantify the compounds.
Don’t Miss: What Does Increased By Mean In Math
Parameters Related To Hplc Separation
Flow Rate
Flow rate shows how fast the mobile phase travels across the column, and is often used for calculation of the consumption of the mobile phase in a given time interval. There are volumetric flow rate U and linear flow rate u. These two flow rate is related by \ref , where A is the area of the channel for the flow, \ref .
What Should I Do First Before Using Hplc

Before the freshly prepare mobile phase is pumped around the HPLC system, it should be thoroughly degassed to remove all dissolved gasses. If the mobile phase is not degassed, air bubbles can form in the high-pressure system resulting in problems with system instability, spurious baseline peaks, etc.
How is HPLC used in water purification?
HPLC is a technique used to separate, identify, and quantify each component in a mixture. Using a pump, a solvent is pressurized through a column to separate and detect these components. Water is used as the solvent to prepare the standards for the mobile phase of HPLC. With HPLC, water purity is critical.
What is column in HPLC?
Columns are the main component in HPLC because the column is responsible for the separation of the sample components. The sample passes through the column with the mobile phase and separates in its components when it comes out from the column. The material filled in the HPLC columns is known as a stationary phase.
You May Like: How To Find Concentration In Chemistry
Who Invented Hplc
Mikhail Semyonovich Tsvet gets credit for inventing liquid column chromatography. In 1901, he presented an adsorption chromatography method for separating plant pigments with petroleum ether in a narrow glass tube filled with calcium carbonate. Tsvet published his work in 1903 and tested 126 different powdered adsorbents. He was the first to use the term chromatography in his papers printed in 1906.
Then 40 years later, in 1941, Archer John Porter Martin and Richard Lawrence Millington Synge published a new type of partition chromatography that used silica gel in columns to keep the water stationary while chloroform flowed through the column to separate amino acids.
This experiment was the beginning of the HPLC development journey, although it took another 30 years before using pumps to push a liquid phase through the packed column.
Today, laboratories worldwide use HPLC to identify and quantify non- and semi-volatile chemical components in liquid samples.
How Is It Used
Broadly speaking, HPLC is used to analyse pharmaceutical products for the ingredients they contain. The method is utilised to separate, quantify and identify the various components along with their quantities within products.
Doing so gives developers a better idea of a drugs properties, with each components quantities affecting the overall performance and strength of a product, for example. However, it also allows them to identify and quantify any impurities within pharmaceutical products.
Most importantly, the method lends itself to the analysis of difficult components, including high molecular weights, thermal instability and compounds which are difficult to volatilise. This makes it suitable for the analysis of drugs in both their pure form and dosage form, such as pills, drinks, powders, injections or inhalers.
Also Check: How To Say Chemistry In Spanish
Isocratic Flow And Gradient Elution
With regard to the mobile phase, a composition of the mobile phase that remains constant throughout the procedure is termed isocratic.
In contrast to this is the so called “gradient elution”, which is a separation where the mobile phase changes its composition during a separation process. One example is a gradient in 20 min starting from 10 % Methanol and ending up with 30 % Methanol. Such a gradient can be increasing or decreasing. The benefit of gradient elution is that it helps speed up elution by allowing components that elute more quickly to come off the column under different conditions than components which are more readily retained by the column. By changing the composition of the solvent, components that are to be resolved can be selectively more or less associated with the mobile phase. As a result, at equilibrium they spend more time in the solvent and less time in the stationary phase, and therefore they elute faster.
How To Read A Chromatogram
The word “chromatogram” means a plot obtained via chromatography. Fig.4 shows an example of a chromatogram. The chromatogram is a two-dimensional plot with the vertical axis showing concentration in terms of the detector signal intensity and the horizontal axis representing the analysis time. When no compounds are eluted from the column, a line parallel to the horizontal axis is plotted. This is called the baseline. The detector responds based on the concentration of the target compound in the elution band. The obtained plot is more like the shape of a bell rather than a triangle. This shape is called a âpeakâ.
Retention time is the time interval between sample injection point and the apex of the peak. The required time for non-retained compounds to go from the injector to the detector is called the dead time .
The peak height is the vertical distance between a peak’s apex and the baseline, and the peak area colored in light blue is the area enclosed by the peak and baseline. These results will be used for the qualitative and quantitative analysis of a sample’s components.
Fig.4ãChromatogram and Related Terms
Read Also: What Is Groynes In Geography
Isocratic And Gradient Elution
A separation in which the mobile phase composition remains constant throughout the procedure is termed isocratic . The word was coined by Csaba Horvath who was one of the pioneers of HPLC.,
The mobile phase composition does not have to remain constant. A separation in which the mobile phase composition is changed during the separation process is described as a gradient elution. One example is a gradient starting at 10% methanol and ending at 90% methanol after 20 minutes. The two components of the mobile phase are typically termed “A” and “B” A is the “weak” solvent which allows the solute to elute only slowly, while B is the “strong” solvent which rapidly elutes the solutes from the column. In reversed-phase chromatography, solvent A is often water or an aqueous buffer, while B is an organic solvent miscible with water, such as acetonitrile, methanol, THF, or isopropanol.
In isocratic elution, peak width increases with retention time linearly according to the equation for N, the number of theoretical plates. This leads to the disadvantage that late-eluting peaks get very flat and broad. Their shape and width may keep them from being recognized as peaks.
In isocratic elution, the selectivity does not change if the column dimensions change that is, the peaks elute in the same order. In gradient elution, the elution order may change as the dimensions or flow rate change.
Pay Attention To Buffers
HPLC will rely on a differential equilibria of the substance you are analyzing, the column, and the solvent. One common solvent is 50% acetonitrile and 50% of 10 mM potassium set to a pH level of 2.5.
To mix this, you want to mix phosphoric acid and potassium dihydrogen phosphate until you get to pH 2.5. Then mix this with an equal amount of acetonitrile.
Always set standard procedures that state buffer formulas and expiry dates. For more tips on how to improve your separations, check out this article.
Recommended Reading: Holt Algebra 2 Powerpoint Presentations
To Test For The Presence Of A Specific Compound/metabolite
Lets say you work for a large animal feed distributor. There have been reports of contaminated grain appearing elsewhere and a new toxin has shown up. You could use HPLC to test for the presence of this toxin in your samples. You could also routinely test your product as part of a quality control program.
What Is Hplc And Its Purpose

High-performance liquid chromatography is an analytical technique to separate, identify, and quantify components in a mixture. It is the single biggest chromatography technique essential to most laboratories worldwide.
How do you perform HPLC?
For setting up the HPLC machine:
What is HPLC used for in chemistry?
is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.
Recommended Reading: Coordinate Geometry In Real Life Examples
Experimental Procedure For Rp
- Install column properly as required.
- Prepare the mobile phase and fill it in the reservoir.
- Prepare samples in different concentrations, as needed.
- Build a method, and fill parameters such as flow rate, the mobile phase composition, wavelength, oven temperature, and program time.
- Create a sequence for samples and save it.
- Purge the mobile phase reservoir by opening the purging valve.
- Gradually increase system flow up to the required flow rate, and wait until the column is saturated and the baseline is corrected.
- As you get a baseline, then inject the sample manually or by auto-sampler via injector .
- After analyzing the sample, study the retention time, tailing factor, capacity factor, and theoretical plates of each peak.
- Repeat the process according to the number of samples.
- Wash the column properly by HPLC grade water, and methanol/ acetonitrile.
principle of hplc