Read A Brief Summary Of This Topic
nuclear fusion, process by which nuclear reactions between light elements form heavier elements . In cases where the interacting nuclei belong to elements with low atomic numbers , substantial amounts of energy are released. The vast energy potential of nuclear fusion was first exploited in thermonuclear weapons, or hydrogen bombs, which were developed in the decade immediately following World War II. For a detailed history of this development, seenuclear weapon. Meanwhile, the potential peaceful applications of nuclear fusion, especially in view of the essentially limitless supply of fusion fuel on Earth, have encouraged an immense effort to harness this process for the production of power. For more detailed information on this effort, seefusion reactor.
This article focuses on the physics of the fusion reaction and on the principles of achieving sustained energy-producing fusion reactions.
Using What Is Fusion In Chemistry
The sun has existed for some five billion decades and is predicted to shine for another five billion years to come. Surely within the world of AI even the conventional definition of life may not be denied. Since then theres been a period of general warming, though it has remained relatively stable over the previous 10,000 decades.
The reason for spondylitis is unknown but theres a strong genetic or family connection. Alternative scoliosis treatment would then be considered anything used to take care of scoliosis that isnt medical. Recently, the borate fusion procedure is also being widely utilized in the lead-zinc-copper market.
Applications Of Nuclear Fusion
We are still at an experimental stage as far as nuclear fusion reactions are concerned.
- Clean: No combustion occurs in nuclear power , so there is no air pollution.
- Less nuclear waste: The fusion reactors will not produce high-level nuclear wastes like their fission counterparts, so that disposal will be less of a problem. In addition, the wastes will not be of weapons-grade nuclear materials as is the case in fission reactors.
If appropriately utilised, nuclear fusion is the answer to the worlds power crisis problem. It is clean and produces a minimal amount of nuclear waste as compared to fission reactions. In addition, the fuel for fusion, Deuterium, and Tritium, are also readily available in nature. Thus, scientists are hopeful that fusion will be a viable alternative power source in the coming centuries.
Recommended Reading: Stitz Zeager College Algebra Book
What Is Nuclear Chemistry
Nuclear chemistry is a sub-discipline of chemistry dealing with the study of changes in the nucleus of atoms of elements. These nuclear changes are a source of nuclear power and radioactivity, and the energy released from the nuclear reactions has far-reaching applications. Nuclear chemistry is also termed radiochemistry, which involves the study of the elements composing the universe, design, and development of radioactive drugs for medicinal uses, and several other scientific applications.
What Is Heat Of Fusion
Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. When studying chemistry, fusion simply has the same definition as melting. In the classroom, you mostly use heat of fusion when a substance is at its melting point or freezing point. In such cases, most think of heat of fusion as a constant.
For instance, water has a heat of fusion of 334 J/g at its melting point of 0°C. This means that, at 0°C, one gram of liquid water must release 334 Joules of energy to completely freeze into ice. Also, one gram of ice must absorb 334 Joules of energy to completely melt at 0°C.
You can calculate the amount of heat energy needed to change a substances phase at its melting point using the following heat of fusion equation:
q = mHf
q: Total change in heat energy
Hf: Heat of fusion of substance
m: Mass of substance
Molar Heat of Fusion
If you know the molar mass of the substance, you can easily convert it into a molar heat of fusion. Water has a molar mass of 18.02 g/mol, so its molar heat of fusion would be 6020 J/mol . Consequently, to calculate the total change in energy, you would instead have to use moles instead of mass:
q = nHf
n: Moles of substance
Read Also: What Does It Mean To Have Chemistry With Someone
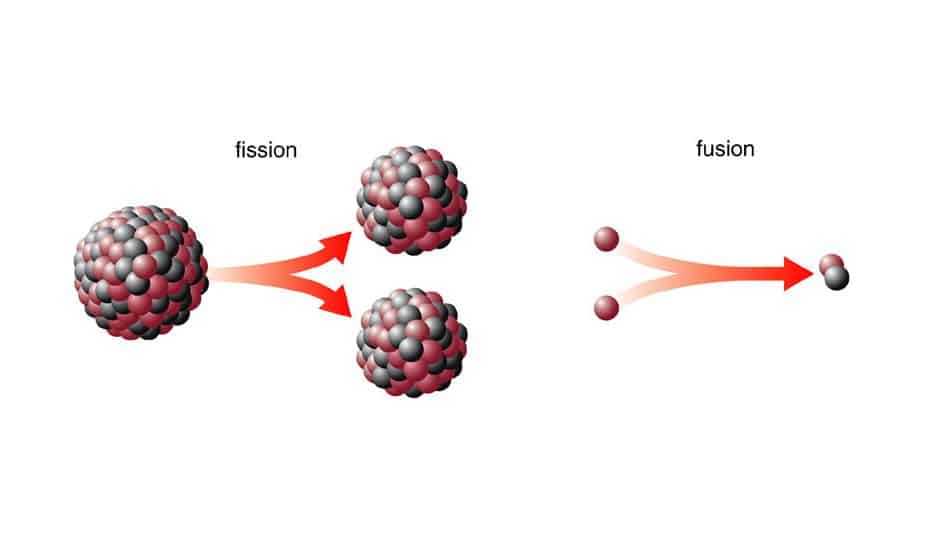
Fission And Fusion: What Is The Difference
All of the energy we produce comes from basic chemical and physical processes.
Thats mostly been accomplished throughout history by burning carbon-based material like wood, coal and gasor by harnessing power from the sun, wind, and water.
Fission and fusion are two physical processes that produce massive amounts of energy from atoms.
They yield millions of times more energy than other sources through nuclear reactions.
You can check out the difference between the two in this video below.
Difference Between Nuclear Fission And Nuclear Fusion
The table below lists the major differences between fusion and fission reactions.
| Nuclear Fission | Nuclear Fusion |
| Nuclear fission is a nuclear reaction that splits a heavy atom into two or more smaller ones. | Nuclear fusion is a nuclear reaction that combines two or more small atoms to form a large atom. |
| It does not occur naturally. | The universe is full of instances of nuclear fusion reactions. Every star uses it to produce energy. |
| It produces a large quantity of energy. | It produces greater energy than the fission reaction. |
| Does not require a lot of energy to split an atom into two. | Requires a lot of heat and pressure for the process to happen. |
To learn the differences in detail, visit the article below:
Recommended Reading: How To Solve Subscripts In Math
What You Must Know About What Is Fusion In Chemistry
Nuclear chemists have to be acquainted with research techniques, be adept at applied mathematics and using laboratory equipment, like a radiation monitor. Impartiality is key in regards to chemical research and experimentation. Ever since then, nuclear studies have been considered extremely sensitive.
This procedure, however, could be familiar to PhD practitioners. Its obvious that although nuclear energy is still one of the most crucial technologies of the present, the future is owned by the renewable resources. Workshops and training camps will aid in doing this.
Nuclear Fission And Fusion
Nuclear fission is the splitting of a heavy nucleus into two lighter ones. Fission was discovered in 1938 by the German scientists Otto Hahn, Lise Meitner, and Fritz Strassmann, who bombarded a sample of uranium with neutrons in an attempt to produce new elements with Z > 92. They observed that lighter elements such as barium were formed during the reaction, and they realized that such products had to originate from the neutron-induced fission of uranium-235:
This hypothesis was confirmed by detecting the krypton-92 fission product. As discussed in Section 20.2, the nucleus usually divides asymmetrically rather than into two equal parts, and the fission of a given nuclide does not give the same products every time.
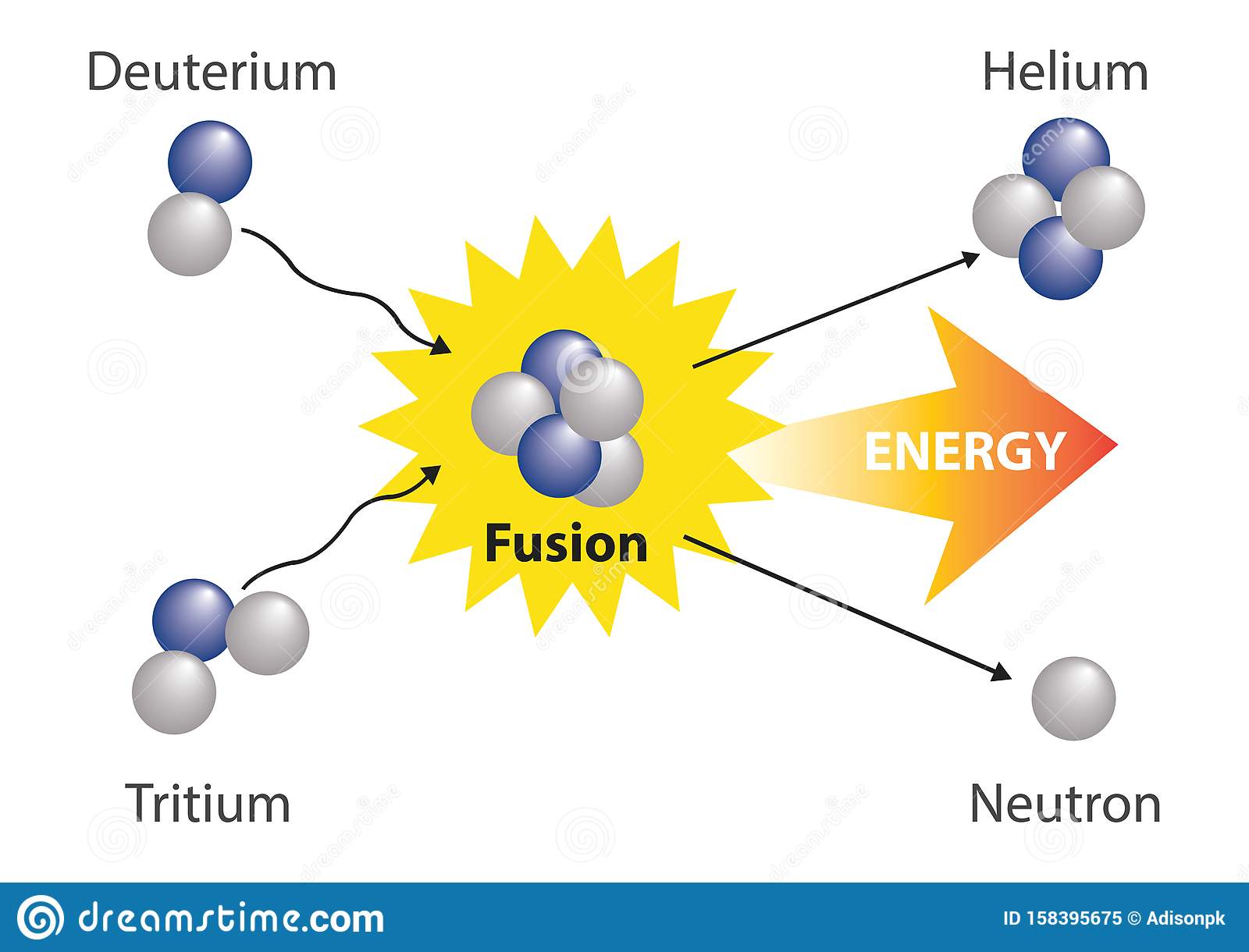
In another reaction, a deuterium atom and a tritium atom fuse to produce helium-4 ), a process known as deuteriumtritium fusion :
To calculate the energy released during mass destruction in both nuclear fission and fusion, we use Einsteins equation that equates energy and mass:
with
- \ is speed of light and
- \ is energy .
Example \: Neutron Induced Fission
Calculate the amount of energy released when the neutron-induced fission of 235U produces 144Cs, 90Rb, and two neutrons:
Given: balanced nuclear reaction
Asked for: energy released in electronvolts per atom and kilojoules per mole
Strategy:
B Calculate the change in mass per mole of 235U. Then use Equation 21.6.3 to calculate the change in energy in kilojoules per mole.
Solution
Exercise \
Solution
You May Like: What Does Additive Mean In Math
How Do Substances Usually Change In Temperature
Typically, when a substance absorbs or releases heat energy, its temperature then changes in response. The amount of temperature change is governed by the substances specific heat, which is a quality intrinsic to a substance and does not depend on how much of the substance you have. The following equation details the relationship between heat energy, specific heat, and temperature.
q = mCT
q: Change in heat
m: Mass of the substance
C: Specific heat of substance
T: Change in Temperature
In this way, you can think of specific heat as the amount of energy needed to change one gram of a substances temperature by one degree Celsius. For instance, the specific heat of gold is 0.128 J/g°C. This means that one gram of pure gold heats by 1°C when it absorbs 0.128 Joules of energy. Conversely, when 0.128 Joules of energy are extracted from the gold, its temperature lowers by 1°C. To learn more about how to use specific heat, check out this article.
Since temperature and heat have a directly proportional relationship, a heat versus temperature graph of a substance without phase changes is linear. When the graph includes phase changes, a strange-looking piecewise slope emerges with flat stretches that correspond to melting and vaporization. As a side note, the phase change between gas and liquid is governed by a heat of vaporization that functions identically to heat of fusion.
How Does Melting Occur
Most solids are packed in a tight crystal lattice with strong intermolecular forces of attraction. On passing heat, the internal binding energy of the crystal lattice gets overcome by the heat energy and the intermolecular forces of attraction weaken. This weakening of force causes instability in the crystal lattice and molecules to separate out from each other and start moving in random directions. The instability in the crystal lattice initiates the melting of a substance. According to the accepted theory of melting, when the temperature of a substance is increased by the virtue of supplying heat or increasing pressure, then the molecules of the substance start vibrating at their places. When the amplitude of the vibration exceeds the inter-atomic distance of the substance, it causes vibrational instability and leads to the melting of the substance.
Read Also: What Is Oscillation In Physics
Fusion Definitions In Physics And Chemistry
Bremsstrahlung Losses In Quasineutral Isotropic Plasmas
The ions undergoing fusion in many systems will essentially never occur alone but will be mixed with electrons that in aggregate neutralize the ions’ bulk electrical charge and form a plasma. The electrons will generally have a temperature comparable to or greater than that of the ions, so they will collide with the ions and emit x-ray radiation of 10â30 keV energy, a process known as Bremsstrahlung.
The huge size of the Sun and stars means that the x-rays produced in this process will not escape and will deposit their energy back into the plasma. They are said to be opaque to x-rays. But any terrestrial fusion reactor will be optically thin for x-rays of this energy range. X-rays are difficult to reflect but they are effectively absorbed in less than mm thickness of stainless steel . This means the bremsstrahlung process is carrying energy out of the plasma, cooling it.
The ratio of fusion power produced to x-ray radiation lost to walls is an important figure of merit. This ratio is generally maximized at a much higher temperature than that which maximizes the power density . The following table shows estimates of the optimum temperature and the power ratio at that temperature for several reactions:
| fuel | |
|---|---|
| 300 | 0.57 |
Recommended Reading: How Did The Geography Of Greece Affect Its Development
What Is Nuclear Fusion
“Nuclear fusion is defined as a process in which lighter nuclei fuse to generate a heavier nucleus, in contrast to nuclear fission. However, such processes can only occur at respectable rates at temperatures of several million degrees, which can only be found in the interiors of stars. Thermonuclear reactions are the name given to such processes . Once a fusion reaction starts, the energy released in the process is enough to keep the temperature constant and the process running.
Two Types Of Fusion Reactions
Fusion reactions are of two basic types: those that preserve the number of protons and neutrons and those that involve a conversion between protons and neutrons. Reactions of the first type are most important for practical fusion energy production, whereas those of the second type are crucial to the initiation of star burning. An arbitrary element is indicated by the notation AZX, where Z is the charge of the nucleus and A is the atomic weight. An important fusion reaction for practical energy generation is that between deuterium and tritium . It produces helium and a neutron and is written D + T He + n.
To the left of the arrow there are two protons and three neutrons. The same is true on the right.
The other reaction, that which initiates star burning, involves the fusion of two hydrogen nuclei to form deuterium :H + H D + + + , where + represents a positron and stands for a neutrino. Before the reaction there are two hydrogen nuclei . Afterward there are one proton and one neutron plus a positron and a neutrino .
Recommended Reading: Geometry Assessment Book Chapter 1 Test B
We Were Willing To Be Wrong We Were Willing To Invest Time And Resources To See If This Might Be An Area Of Useful Research In Our Quest To Eliminate Pollution
Thomas F. Darden, CEO, Cherokee
The other main branch of the field uses a nickel-hydrogen setup, which can produce greater than 400 times as much energy as it uses. Nagel likes to compare these LENR technologies to that of the International Thermonuclear Experimental Reactor, a multination high-temperature fusion experiment based on well-understood physicsmerging deuterium and tritiumbeing carried out in southern France. At a cost exceeding $20 billion, this 20-year project has set a goal of generating 10 times as much energy as it consumes.
Nagel says the LENR field continues to grow internationally, and the biggest hurdles remain inconsistent results and lack of funding. For example, some researchers report that a certain threshold must be reached for a reaction to start. The reaction may require a minimum amount of deuterium or hydrogen to get going, or the electrode materials may need to be prepared with a specific crystallographic orientation and surface morphology to trigger the process. The latter is a common issue with heterogeneous catalysts used in petroleum refining and petrochemical production.
Nagel acknowledges that the business side of LENR has had problems too: Prototypes being developed have been relatively crude, he says, and there has yet to be an LENR-based company to offer a working product or make any money.
Rossis E-Cat
Scientific legacies
Time will tell
The Good The Bad And What Is Fusion In Chemistry
Such issues mean that nuclear energy isnt as popular as more conventional procedures of getting energy, like the use of fossil fuels. Moreover, its also used to produce musical instruments like flute and saxophones. It demonstrates that the energy stored in even a little quantity of mass is tremendous.
Insufficient power supply is just one of the primary causes of crippling economies. There are more than 2,000 hydro power plants in the United States, which makes it the most significant source of energy in the nation. So it takes a lot of energy to visit a gas instead of as much energy to visit a liquid.
Don’t Miss: What Is The Unit For Acceleration In Physics
Which Definition To Use
Because fusion can refer to so many processes, it’s a good idea to use the most specific term for a purpose. For example, when discussing the combination of atomic nuclei, it’s better to refer to nuclear fusion rather than simply fusion. Otherwise, it’s usually obvious which definition applies when used in the context of a discipline.
Fusion Definition In Biology And Medicine
Don’t Miss: Common Core Algebra 2 Unit 13 Lesson 9 Answers
Applications Of Nuclear Chemistry
Following are the Applications of Nuclear Chemistry:
- Nuclear waste is radioactive waste, which means it emits radiation on its own. It mainly comes from the by-products of nuclear processes in medical and scientific applications.
- A nuclear reactor is a device that generates nuclear reactions and controls the chain reaction to release a significant amount of steady heat, resulting in the production of energy.
- The term “nuclear weapon” refers to a device that uses a nuclear reaction for destructive purposes.
- The procedure of establishing the age of a sample by comparing the quantity of C-14 left to the known half-life of 5,730 years is known as radiocarbon dating. This method works because organisms that are living regularly renew their C-14 supply through respiration, ensuring that they have a steady supply of the isotope.
- MRI scans, CT scans, and X-rays for diagnosis.
- In space missions, radioisotope thermal generators are used to generate electricity.
- Food is irradiated with gamma rays to keep it fresh and extend its shelf life.
- Cancer patients are treated with radioactive iodine.
- Medical instruments are sterilized.