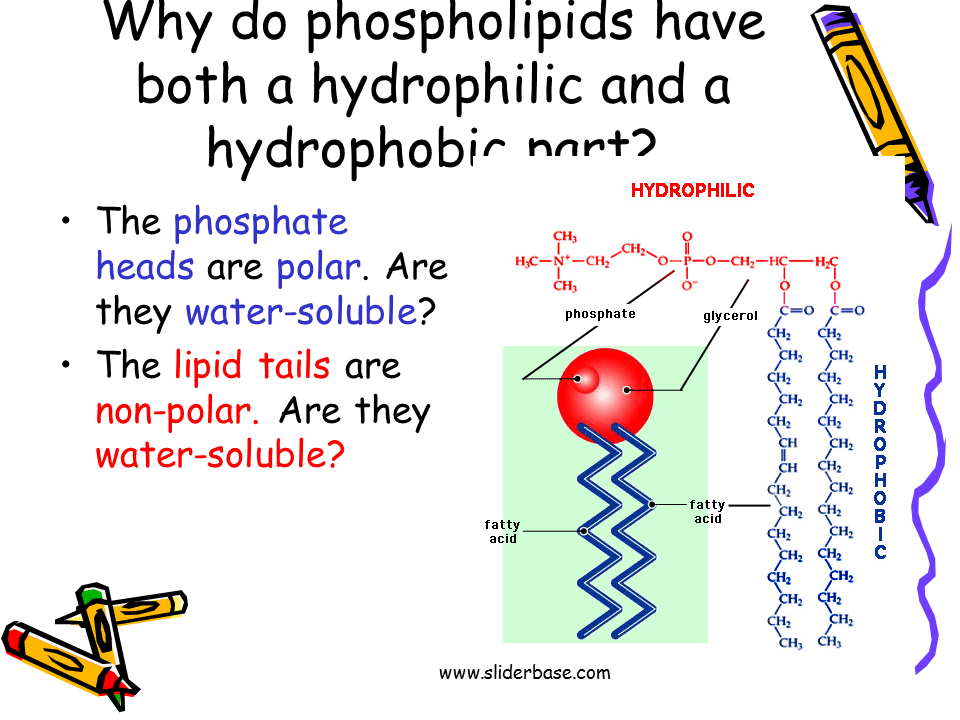
Hydrophilic And Hydrophobic Properties
Figure 4.3. FESEM of multi-walled carbon nanotubes/unidirectional basalt fiber/epoxy composites showed agglomeration .
The hydrophilic and hydrophobic properties are related to water absorption, which can be calculated as in Eq. . A higher percentage of water content results from higher hydrophilic properties and a lower percentage of water absorption is proportional to high hydrophobic properties, and vice versa.
The contact angle test is commonly used to determine the hydrophilicity and hydrophobicity on the surface of a material. This test could be performed by using different liquids, the most common of which is a water droplet. The degree of the angle on the surface of materials is calculated, as explained in Fig. 4.4. Fig. 4.5 shows an example of the contact angle test using a water droplet and ethanol on the surface of a polycarbonate nanoparticle composite.
Figure 4.4. Different properties of contact angle test.
Figure 4.5. Contact angle test on polycarbonate nanoparticle composite .
Kimmo Henttinen, Tommi Suni, in, 2015
How Do Detergents Work Biology
How do detergents work? Soaps and detergents are made from long molecules that contain a head and tail. These molecules are called surfactants the diagram below represents a surfactant molecule. The detergent molecules also help to make the washing process more effective by reducing the surface tension of the water.
Why Is Soap Amphiphilic
Due to their opposite polarity, water by itself cannot penetrate grease or oil. Soap molecules are amphipathic and thus have both properties of non-polar and polar at opposite ends of the molecule. The oil is a pure hydrocarbon so it is non-polar. The non-polar hydrocarbon tail of the soap dissolves into the oil.
Recommended Reading: How To Find Ksp Chemistry
The Water Race: Hydrophobic & Hydrophilic Surfaces
Audience: High School
Nonpolar molecules that repel the water molecules are said to be hydrophobic molecules forming ionic or a hydrogen bond with the water molecule are said to be hydrophilic. This property of water was important for the evolution of life. Hydrophobic interaction plays the most critical roles in the formation of the lipid bilayer of the cell membrane and the folding of proteins and nucleic acids therefore, hydrophobic interaction is the foundation for the existence of life.
A self-assembled monolayer is a layer of organic molecules formed spontaneously on a solid substrate. One end of the organic molecule binds to the solid surface via a covalent bond while the other end points outwards. Because the exposed end of the SAM determines the surface properties of the SAM modified substrate, we can alter a hydrophobic surface into a hydrophilic surface by carefully selecting the SAM forming molecules.
This lesson requires the use of special chemicals which can be ordered from standard supply houses.
How Do Micelles Help Clean Clothes
When we wash clothes, the hydrophilic end attaches with the water while the hydrophobic end attaches with the dirt. thus a micelle is formed. When we scrub the cloth, the dirt is pulled off as the micelle gets washed away with water taking the dirt with it. Micelles do not dissolve in water but remain as colloids.
You May Like: Paris Jackson’s Biological Father
What Does Polar In Biology Mean
Polar in biology means
Correspondingly, whats a polar molecule in biology?
A polar molecule is a chemical species in which the distribution of electrons between the covalently bonded atoms shouldnt be even. Polarity is an outline of how totally different poles of a molecule are. When bonded to a different atom, the atom with the upper electronegativity will have a tendency to draw extra electrons.
One can also ask, what does polar imply in science? The association or geometry of the atoms in some molecules is such that one finish of the molecule has a optimistic electrical cost and the opposite aspect has a unfavourable cost. If this is the case, the molecule is referred to as a polar molecule, that means that it has electrical poles.
In addition to, what does polar or nonpolar imply?
Polar molecules happen when theres an electronegativity distinction between the bonded atoms. Nonpolar molecules happen when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a bigger molecule cancel one another out.
What does hydrophobic imply in biology?
Hydrophobic Definition. Hydrophobic actually means the concern of water. Hydrophobic molecules and surfaces repel water. Hydrophobic liquids, similar to oil, will separate from water. Hydrophobic molecules are often nonpolar, that means the atoms that make the molecule do not produce a static electrical discipline.
Contents Inside :
Whats The Difference Between The Two
According to these straight definitions, we can see that these two terms are opposites. Something defined as hydrophilic is actually attracted to water, while something that is hydrophobic resists water. This means when hydrophobic items come in contact with liquids, water is encouraged to bead up and roll off the surface- almost pushing it away like a magnet pushes away metal objects.
A great example of something that is hydrophilic is self-cleaning glass. This special glass has been engineered and coated with a nano-sized, thin-film. Instead of allowing water to form into water droplets that bead up and roll off of the glass, this cool nanotechnology helps tiny water molecules to glide over the surface in a sheet, washing dirt or other debris away.
Read Also: Homework 4 Angle Addition Postulate Answers
What Is The Definition Of Hydrophilic In Biology
4.6/5Hydrophilic
Hydrophobic Definition. Hydrophobic literally meansthe fear of water. Hydrophobic molecules and surfaces repel water. Hydrophobic liquids, such as oil, will separate from water. Hydrophobic molecules are usually nonpolar, meaning the atoms that make the molecule do not produce a static electric field.
Furthermore, what is an example of a hydrophilic? Examples of hydrophilic liquids include ammonia, alcohols, some amides such as urea and some carboxylic acids such as acetic acid.
Beside this, what does hydrophobic and hydrophilic mean in biology?
Nonpolar molecules that repel the water molecules are said to be hydrophobic molecules forming ionic or a hydrogen bond with the water molecule are said to be hydrophilic. This property of water was important for the evolution of life.
What does amphiphilic mean in biology?
Definition of amphiphilic. : of, relating to, or being a compound consisting of molecules having a polar water-soluble group attached to a water-insoluble hydrocarbon chain also : being a molecule of such a compound.
Hydrophilic Membranes And Coatings
Hydrophilic membranes are basically nonporous films that breathe via osmotic potential by adsorbing and desorbing the vapour molecules and then conveying them to the other side, much like cells in human body allow diffusion of monomolecular and ionic species through their membrane walls. The driving force for water vapour transmission, however, is a difference in water vapour pressure between the two faces of the membrane. Breathability in hydrophilics is therefore an adsorptiondiffusiondesorption process, accelerated by hydrogen bonds between water molecules and functional groups built into the molecular chains. Fig. 2.3 shows a schematic diagram of the mechanism of vapour transport through a hydrophilic polymer. The water vapour molecules first adsorb on the surface with higher vapour concentration, occupy free volume between the polymer molecular chains, and move across the membrane without interacting chemically with the polymer. The amorphous regions act like intermolecular pores, allowing water vapour molecules to pass through but preventing the penetration of liquid water owing to the solid nature of the membrane. In case of active hydrophilic groups, vapour molecules not only fill the free volume but also interact with the active hydrophilic groups.2 They then diffuse across by dissolving in the polymer membrane, which is known as activated diffusion. Upon arriving at the opposite surface of the membrane, they are desorbed into the surrounding air space.18
You May Like: Elton John Kids Adopted
Pressure Gravity And Matric Potential
What Does Integral Mean In Biology
An integral protein, sometimes referred to as an integral membrane protein, is any protein which has a special functional region for the purpose of securing its position within the cellular membrane. It does so with regions of specific amino acids which are attracted to the middle of the plasma membrane.
Recommended Reading: What Is The Molecular Geometry Of Ccl4
How Do You Use Lysis In A Sentence
Use lysis in a sentence | lysis sentence examples
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Read Also: Fsa Warm Ups Grade 5 Answer Key
Main Difference Hydrophobic Vs Hydrophilic Molecules
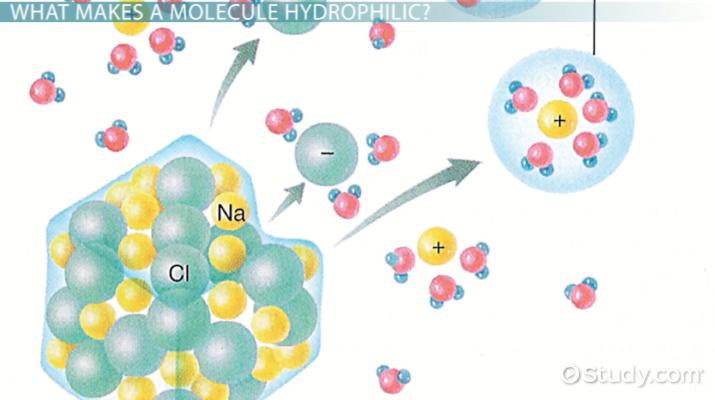
Water is a well-known solvent for the dissolution of most of the compounds we know. But all compounds in nature do not mix with water. The substances that can mix with water are called hydrophilic substances the substances that cannot mix with water are known hydrophobic substances. This happens mainly due to the polarity of water molecules. Nonpolar compounds cannot dissolve in a polar solvent. Here, we should consider the fact like dissolves like. Polar compounds can dissolve in polar solvents. Nonpolar compounds dissolve in nonpolar solvents. Therefore, hydrophilic substances should be polar in order to dissolve in water. The main difference between hydrophobic and hydrophilic molecules is that hydrophobic molecules are nonpolar whereas hydrophilic molecules are polar.
What Is Plaster Of Paris Class 11
Uses of Plaster of Paris 1) It used for producing moulds for pottery and ceramics and casts of statues and busts. 2) It is used for making statues ,models and other decorative materials. 3) It is used in surgical bandages used for plastering broken or fractured bones of the body and for preparing blackboard Chalk.
You May Like: What Does Abiotic Mean In Biology
Does Plaster Of Paris Catch Fire
PLASTER OF PARIS is non-flammable and non-combustible. Has generally low chemical reactivity but can act as an oxidizing agent under extreme conditions. Decomposes at high temperature to generate toxic oxides of sulfur. Reacts exothermically but slowly with moisture in the air or water to form gypsum CaSO4.
What Are The Types Of Integral Proteins
According to their their relationship with the bilayer, integral membrane protein can be classified two primary types: integral polytopic proteins and Integral monotopic proteins. Integral polytopic proteins are also known as transmembrane proteins which can span across the membrane at least once (Fig.
Don’t Miss: What Is The Molecular Geometry Of Ccl4
What Is Mean By Hydrophobic
hydrophobichydrophobic
Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds.
One may also ask, what does hydrophilic mean in biology? adjective. Having an affinity for water capable of interacting with water through hydrogen bonding hygroscopic. Supplement. Hydrophilic molecules typically have polar groups enabling them to readily absorb or dissolve in water as well as in other polar solvents.
Additionally, what makes something hydrophobic or hydrophilic?
The Water Race: Hydrophobic& Hydrophilic Surfaces. Nonpolar molecules that repel the water molecules are said to be hydrophobic molecules forming ionic or a hydrogen bond with the water molecule are said to be hydrophilic. This property of water was important for the evolution of life.
What are hydrophobic plants?
Surfaces that repel water, like the leaves of the Colocasia plant, are called hydrophobic. Hydro- is a Greek root word that means water.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Also Check: Geometry Dash Eric Van Wilderman
Why Is Hydrophilic Important
Hydrophilic is a combination of the prefix hydro-, meaning water, and the suffix -philic, which indicates that something has an affinity for or love of something else. Of course, hydrophilic things dont actually love waterbut they are known for combining or interacting with it. Water is essential to all living thingswhats not to love?
When something, such as a chemical, is attracted to or easily travels through water, it is described as hydrophilic. Its the kind of word youre likely to see in scientific journals, but its also used in other fields, such as agriculture and industrial manufacturing.
So, what makes water so lovable? Well, water is a simple liquid that is made of molecules consisting of one oxygen atom and two hydrogen atoms . The ends of the water molecules also have small electrical charges with the oxygen side being negative and the hydrogen side being positive. This makes water polar. Other polar things bond with water molecules in the same way that opposite ends of magnets will stick to each other. When something bonds with water, a variety of different things can happen. Hydrophilic sugars or salts might dissolve into the water. A hydrophilic alcohol might mix together with the water, or a hydrophilic sponge might absorb the water into itself. But water isnt loved by everythingthings that tend not to be attracted to or combine with water are instead called hydrophobic.
Examples Of Hydrophilic In A Sentence
hydrophilic Harper’s BAZAARhydrophilic Forbeshydrophilic Outside Onlinehydrophilic Field & Streamhydrophilic Smithsonian Magazinehydrophilic Ars Technicahydrophilic Ars Technicahydrophilic Ars Technica
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘hydrophilic.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
You May Like: What Is The Molecular Geometry Of Ccl4
How Do Detergents Solubilize Membrane Proteins
Detergent monomers solubilize membrane proteins by partitioning into the membrane bilayer. The solubilization buffer should contain sufficient detergent to provide greater than 1 micelle per membrane protein molecule to help ensure that individual protein molecules are isolated in separate micelles.
Storage Of Solids In Bulk
10.4.1Outdoor bulk storage
A hydrophilic-products processing plant needs a large storage area to keep its products dry during the low demand season. Long experience achieved in many plants proved that hydrophilic material, such as ammonium nitrate, can be safely stored outside in bulk. This approach may be applied to similar bulk materials.
The outdoor bulk storage bins are considered as easy and economical to construct and simple in operation.
10.4.2Designing and outdoor storage bin
In design phase many factors have to be considered such as: storage capacity, rate of stockpiling, operation, the materials physical and chemical properties, shape of the stockpile, area required, available capital investment, operating cost, product protection, etc.
Mohamad Nurul Azman Mohammad Taib, Nurhidayatullaili Muhd Julkapli, in, 2019
Read Also: Algebra Nation Section 7: Exponential Functions Answers
How Do Xerophiles Survive
While liquid water is absolutely essential for the growth and reproduction of all terrestrial life, certain organisms can tolerate periods of extreme desiccation: the xerophiles. They survive by entering a state of anhydrobiosis, in which minimal water remains and cells metabolic activity enters dormancy.
What Is Hydrophilic Quizlet
hydrophilichydrophilichydrophobic
Hydrophilic Definition. A hydrophilic molecule or substance is attracted to water. Water is a polar molecule that acts as a solvent, dissolving other polar and hydrophilic substances. In biology, many substances are hydrophilic, which allows them to be dispersed throughout a cell or organism.
Similarly, what is meant by hydrophobic and hydrophilic? Hydrophilic means water loving hydrophobic means resistant to water. 2. Hydrophilic molecules get absorbed or dissolved in water, while hydrophobic molecules only dissolve in oil-based substances.
Besides, what is mean by hydrophobic?
The word hydrophobic comes from the Greek roots hydro- and -phobia . The word hydrophobic describes the fact that nonpolar substances don’t combine with water molecules. Water is a polar molecule, which means that it carries a partial charge between its atoms.
What does hydrophilic mean in biology?
adjective. Having an affinity for water capable of interacting with water through hydrogen bonding hygroscopic. Supplement. Hydrophilic molecules typically have polar groups enabling them to readily absorb or dissolve in water as well as in other polar solvents.
Also Check: Definition Of Abiotic In Science
What Are Hydrophobic Molecules
Hydrophobic molecules are molecules that do not dissolve in water. Therefore, these molecules repel water molecules. These hydrophobic molecules are called hydrophobes. The hydrophobicity describes how much hydrophobic a molecule is.
Hydrophobic molecules are hydrophobic due to their non-polarity in other words, hydrophobic molecules are nonpolar. Therefore, hydrophobic molecules are often composed of long chain hydrocarbon groups which can make a molecule nonpolar.
Figure 1: Hydrophobic
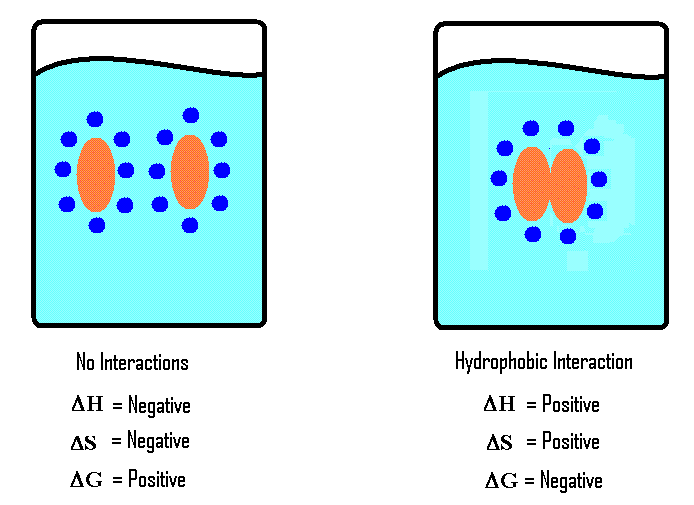
When hydrophobic molecules are added to water, these molecules tend to form micelles, which look like clumps, in order to have a minimal contact with water. However, water molecule arranges around these clumps to form a cage. When this clump is forming, the hydrogen bonds between water molecules are broken down, making space for the clump. This is an endothermic reaction since chemical bonds are broken down. Moreover, the formation of clumps causes the entropy of the system to decrease.
According to thermodynamic relations,
Where G is the Gibbs free energy
H is the change in enthalpy
T is the temperature
S is the change in entropy.