Is Pbs The Same As Saline
PBS closely mimics pH, osmolarity and ion concentration of cells while saline solution lacks buffering capacity and has lower pH than cells. That makes PBS more adequate for washing cells, however, if a quick wash is required prior to tripzin for example you can use saline without problems.
Is PBS aqueous or solid?
Physical properties: Lead sulfide is a black crystalline solid or a silver powder. Its density is 7.5 g mL-1. Its melting point is 1114 ºC and its boiling point is 1281 ºC. It is insoluble in water and soluble in nitric acid hot and hydrochloric acid.
Is PbS aqueous or solid?
What Is Pbs Chemistry
Phosphate-buffered saline is a buffer solution commonly used in biological research. It is a water-based salt solution containing disodium hydrogen phosphate, sodium chloride and, in some formulations, potassium chloride and potassium dihydrogen phosphate. The buffer helps to maintain a constant pH.
How is PbS formed?
Following the creation of the Public Broadcasting Act , the government-funded Corporation for Public Broadcasting was established, and in 1969 it founded the Public Broadcasting Service as a successor to NET. The PBS broadcast network debuted in 1970.
What is the correct chemical name for Cu2O?
Copper oxideCopper oxide or cuprous oxide is the inorganic compound with the formula Cu2O.
Diluting A 10x Solution To Make 1x Pbs
10X is a concentrated or stock solution, which may be diluted to make a 1X or normal solution. A 5X solution must be diluted 5 times to make a normal dilution, while a 10X solution must be diluted 10 times.
To prepare a 1 liter working solution of 1X PBS from a 10X PBS solution, add 100 ml of the 10X solution to 900 ml of water. This only changes the concentration of the solution, not the gram or molar amount of the reagents. The pH should be unaffected.
Read Also: Scratch Mit Edu Geometry Dash
Curcumin Release Profile From Sf Nanoparticles
One milligram of curcumin-loaded SF nanoparticles was suspended in 1 mL of PBS in 2-mL Eppendorf tubes and incubated at 37°C in air containing 5% CO2 and 90% humidity. At predetermined time points , ethanol was added to each tube to dissolve any free curcumin released from the nanoparticles. Nanoparticle solutions were centrifuged at 14,000 rpm for 5 minutes and the curcumin content within the supernatant was measured at 590 nm using UV spectroscopy. Curcumin standards were prepared in a solution of 50% ethanol in water and used for reference.
P. Aebischer, … S.R. Winn, in, 1991
Sterilization And Storage Of Pbs Solution
Sterilization isn’t necessary for some applications, but if your are sterilizing it, dispense the solution into aliquots and autoclave for 20 minutes at 15 psi or use filter sterilization.
Phosphate-buffered saline may be stored at room temperature. It may also be refrigerated, but 5X and 10X solution may precipitate when cooled. If you must chill a concentrated solution, first store it at room temperature until you are certain the salts have completely dissolved. If precipitation does occur, warming the temperature will bring them back into solution. Shelf life of refrigerated solution is 1 month.
Read Also: Is Physics On The Dat
What Pbs Stands For
What is the name of the compound with the formula PBS?
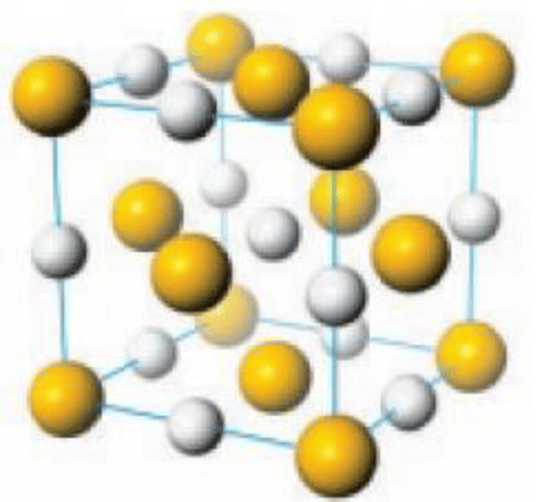
Lead sulfide is an inorganic compound with the chemical formula PbS. It is also known as galena, which is the principal ore and important compound of lead.
What is the name of the compound PBS2?
PbS2 or H4PbS2. Synonyms. Lead sulfide. Molecular Weight. 275 g/mol. Component Compounds. CID 5352425 CID 402 Date s.
Tunel Staining Of Atherosclerotic Lesions
Add 100 l of protease K solution to each slide and incubate for 30 min at 37 °C. Then, rinse 2× in PBS and hold in PBS solution. First process positive control: Add a 50-L DNase solution and leave for 10 min at room temperature. Wash 2× in PBS. Next, dry the area around all samples, including positive and negative control. Prepare Roche TUNEL reaction mix as per the manufacturer’s instructions. This is done by mixing 50 L of vial 1 + 450 L vial 2 . This is for nine samples and a positive control. TUNEL staining is performed by adding 50 l of enzyme mixture to each sample and to positive control. Do not add TUNEL reaction mixture to negative control. Incubate all samples plus controls 60 min at 37 °C in a dark, humidified incubator. Rinse the slides in PBS 3× . Add Hoechst solution to each slide for 5 min. Wash in PBS two times and coverslip using GVA mounting solution. Check for apoptosis per microscopy .
Ting Jiang, Scott T. Laughlin, in, 2020
Don’t Miss: What Is Random Sampling In Psychology
Formation Basic Properties Related Materials
Addition of hydrogen sulfide or sulfide salts to a solution containing a lead salt, such as PbCl2, gives a black precipitate of lead sulfide.
- Pb2+ + H2S PbS + 2 H+
This reaction is used in qualitative inorganic analysis. The presence of hydrogen sulfide or sulfide ions may be tested using “lead acetate paper.”
Like the related materials PbSe and PbTe, PbS is a semiconductor. In fact, lead sulfide was one of the earliest materials to be used as a semiconductor. Lead sulfide crystallizes in the sodium chloride motif, unlike many other IV-VI semiconductors.
Since PbS is the main ore of lead, much effort has focused on its conversion. A major process involves smelting of PbS followed by reduction of the resulting oxide. Idealized equations for these two steps are:
- 2 PbS + 3 O2 2 PbO + 2 SO2
- PbO + C Pb + CO
Scanning Electron Microscopy Of Cell Growth On Ha
The fixed samples were washed with PBS , stained with 1% osmium tetroxide for 30 min, then washed three times with distilled water. The samples were then dehydrated by sequential incubations in 50%, 70%, 80%, and 90% in ethanol and, finally, washed in 100% ethanol three times, then dried with a Pelco CPD2 CO2 Critical Point Dryer. The dried samples were mounted and gold coated using a Pelco SC-6 Sputter . The SEM analysis was performed using a Hitachi S-2460N, with 10 kV of accelerating voltage. The cell concentration was quantitated by counting four to five fields per die, on the corners and middle, and then the results were averaged. A field is calculated to be 425 m×559 m or 237,447 m2 a field is calculated to be 385 m×295 m or 113,575 m2 and a field is calculated to be 375 m×290 m or 108,750 m2.
K.E. SWINDLE-REILLY, N. RAVI, in, 2010
Read Also: What Does Shielding Mean In Chemistry
How To Prepare Phosphate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
PBS or phosphate-buffered saline is a buffer solution that is particularly valuable because it mimic the ion concentration, osmolarity, and pH of human body fluids. In other words, it’s isotonic to human solutions, so it’s less likely to cause cell damage, toxicity, or unwanted precipitation in biological, medical, or biochemical research.
How To Make Phosphate Buffered Saline
- University of Guelph
- University of Waterloo
Phosphate buffered saline is a buffer solution that’s commonly used for immunohistochemical staining and it’s often used in biological research. PBS is a water-based salt solution containing sodium hydrogen phosphate, sodium chloride and, in some cases, potassium chloride and potassium dihydrogen phosphate.
Read Also: College Algebra Graphing Square Root Functions
A Recipe For Pbs Buffer
You can prepare PBS in several ways. There are multiple formulas. Some of them don’t contain potassium, while others contain calcium or magnesium.
This recipe is relatively easy. It’s for 10X PBS stock solution . However, you can also make a 1X stock solution, or begin with this 10X recipe and dilute it to 1X. The whole process takes about 10 minutes and an option to add Tween is also provided.
Characterisation Of Immobilised Fibronectin Layers And Displacement Of Predeposited Fibronectin In Serum

Fibronectin was immobilised to the polymer films from stagnant PBS solutions containing 50 g/ml of fibronectin for one hour. The surface concentrations of fibronectin on the compared substrates, as shown in Fig. 13.12, were measured by high pressure liquid chromatography based amino acid quantification after acidic hydrolysis. The obtained surface concentrations of about 450 ng/cm2 were found to be rather similar on the different substrates and may represent a monolayer coverage with different molecular orientations of the protein on the smooth substrates. A slightly reduced surface concentration of fibronectin was found on the hydrophilic PPMA-p.
13.12. Immobilised amounts of FN on the compared variants of copolymer surfaces determined by amino acid quantification.
Various amounts of saturated fibronectin adsorption layers on polymer substrates are reported in the literature.21,62,63 Values of about 400ng/cm2 have often been considered to correspond to a monolayer coverage at flat surfaces.64 The conditions applied for fibronectin immobilisation for the cell culture experiments in this study were verified by HPLC analysis using different solution concentrations of fibronectin with an incubation time of one hour. Almost saturated layers of fibronectin were obtained on the polymer substrates immersed in solutions containing 50g/ml fibronectin .
13.13. Immobilised amounts of FN on POMA-c surfaces for different FN solution concentrations determined by HPLC analysis.
Recommended Reading: Geometry Unit 5 Study Guide Answers
Effect Of Pbs Concentration On Sf Scaffold Properties
Particle size of SF solutions ) varied with different PBS concentrations . SF solution with no PBS had a mean particle size of 202.6 nm, whereas SF solutions with final PBS concentrations of 0.1× PBS and 1× PBS had mean particle sizes of 400.0 and 252.7 nm, respectively. Scaffolds fabricated with SF solutions with or without PBS had primarily porous structures . The presence of PBS in SF solutions did not significantly change the porosity, fibril size, or fibril density in SF scaffolds . However, the increase in PBS concentration in SF solution decreased the pore size in SF scaffolds from 212.0±23.7 µm for the no PBS group to 146.4±20.5 µm for the 1× PBS group . A change in ionic concentration of SF solution affected the mechanical properties of SF scaffolds . SF scaffolds made from SF solution with a low PBS concentration showed lower UTS and E compared with SF scaffolds made from SF solution without PBS or with a high PBS concentration .
Table 5.4. Effect of PBS Concentration on Particle Size, Scaffold Structure, and Scaffold Mechanical Properties
| No PBS |
|---|
Pier Giorgio Righetti, Egisto Boschetti, in, 2013
Phosphate Buffered Saline Pbs Sterile 500 Ml
- Make sure this fitsby entering your model number.
- Cell Suspension Tested
- This item: Phosphate Buffered Saline, PBS , Sterile, 500 mL$30.00Ships from and sold by American BioInnovations.Get it
- Only 19 left in stock – order soon.Sold by ADVANGENE and ships from Amazon Fulfillment.Get it as soon as Wednesday, Oct 26
Also Check: What Is Gametes In Biology
How To Make Pbs Buffer