Chemical Synthesis By Oxidation
Chemical synthesis is a top-down indirect graphene synthesis method, and it is the first method that demonstrated graphene synthesis by a chemical route. In the year 1962, Boehm et al. first demonstrated monolayer flakes of reduced graphene oxide, which was recently acknowledged by the graphene inventor Andre Geim. The method involves the synthesis of a graphite oxide by oxidation of graphite, dispersing the flakes by sonication, and reducing it back to graphene. There are three popular methods available for GO synthesis: the Brodie method , Staudenmaier method , and Hummers & Offeman method . All three methods involve oxidation of graphite using strong acids and oxidants. The degree of oxidation can be varied by the reaction conditions , stoichiometry, and the type of precursor graphite used as a starting material. The process flow chart of the chemical synthesis of graphene is shown in a schematic in Fig. 16.21.
Figure 16.21. The process flow chart of graphene synthesis derived from graphite oxide.
All of the previously mentioned processes comprise the chemical approach for synthesizing graphene. Direct graphene synthesis using electrochemical methods was reported by Liu et al. . The method is environment friendly and leads to the production of a colloidal suspension of imidazolium ion-functionalized graphene sheets by direct electrochemical treatment of graphite.
Pouran Pourhakkak, … Mehrorang Ghaedi, in, 2021
Synthesis Of Sodium Carbonate Na2co3
Sodium carbonate is now exclusively manufactured by the Solvey process. In this process carbon dioxide and ammonia are passed into a cold saturated solution of sodium chloride. In the reactions which occur sodium hydrogen carbonate is formed which is only very slightly soluble in the presence of sodium ions, is almost completely precipitated. It is removed by filtration and ignited to produce sodium carbonate.
The ingredients of this process are readily available and inexpensive. These are salt brine , ammonia and limestone . In this process, CaCl2 is an important by-product obtained.
The reactions can be represented by the following equation.
2NH3 + H2O + CO2 2CO32CO3 + H2O + CO2 2NH4HCO3
Addition of common salt to the solution containing NH4+ and HCO3 results in the precipitation of NaHCO3 which is least soluble. It is then filtered off.
NH4HCO3 + NaCl NH4Cl + NaHCO3
Sodium bicarbonate is then heated to give Na2CO3.
2NaHCO3 Na2CO3 + CO2 + H2O
The CO2 gas evolved can be reused again.
Anhydrous sodium carbonate is dissolved in water and recrystallizes to get washing soda crystals containing 10 molecules of water of crystallization.
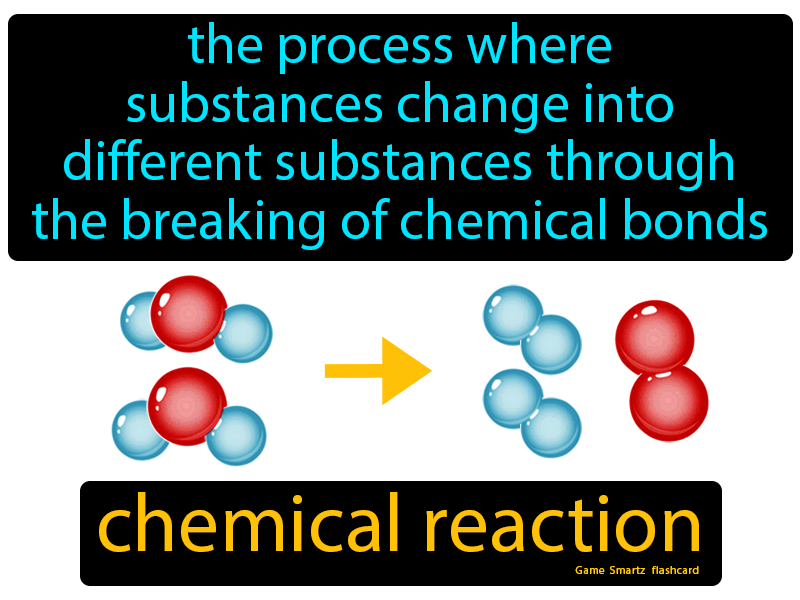
Synthesis And Decomposition Reactions
Synthesis and decomposition are two types of redox reactions. Synthesis means to make something, whereas decomposition means to break something. The reactions are accompanied by chemical and energy changes.
Synthesis Reactions
Synthesis reactions are also called combination reactions. It is a reaction in which two or more substances combine to form a complex substance. Synthesis reactions are generally represented as: A + B AB or A + B C. The formation of nitrogen dioxide is a synthesis reaction: 2 NO + O2 2 NO2 .
In synthesis reactions, the reactants could be all elements , or a combination of an element and a compound , or all compounds .
1) C + O2 CO2 2) 2 CO + O2 2 CO2 3) 2 CaO + 2 H2O 2 Ca2
A combination reaction between a metal and a nonmetal always produces an ionic solid. For example, the formation of sodium chloride or table salt from sodium and chlorine is a combination reaction: 2 Na + Cl2 2 NaCl .
A synthesis reaction is generally accompanied by the release of energy. In the above example of sodium chloride, 787 kJ of heat energy is released.
Oxygen was first discovered by the scientist Joseph Priestley, in 1774, by heating mercury oxide with a burning glass. The reaction was a result of decomposition. Priestley had broken down mercury oxide with heat into its elements. The reaction is represented as: 2 HgO 2 Hg + O2
The reaction is represented as: 2 NaN3 2 Na + 3 N2
1) 2 Al2O3 4 Al + 3 O2 2) 2 KClO3 2 KCl + 3 O2 3) NH4Cl NH3 + HCl
Also Check: Chapter 4 Test Form 2c
Quick Facts About Asymmetric Synthesis
The phenomenon of asymmetric synthesis is applied to multiple settings in which we live and that surround us.
-
The progress of the drug industry is solely dependent on the workings of asymmetric synthesis.
-
Variations in the reaction patterns of all the living systems are attributed to different enantiomers, this is possible because living beings possess a high degree of the property, chirality.
-
The basis of amino acids and sugars is rooted back in the existence of a mere enantiomer.
-
New developments in the field of asymmetric synthesis are taking place. One such technological development is known as asymmetric organocatalysis.
This is exactly what the fundamentals of asymmetric catalysis are.
Similarities Between Synthesis And Retrosynthesis
- Synthesis and retrosynthesis are two processes in the construction of a particular organic compound.
- Both use simple precursor molecules in order to synthesize a complex organic compound through a series of chemical reactions in organic chemistry.
- Here, the precursors can be either commercially available or natural molecules.
- The construction of organic compounds is important for industrial purposes, especially in the pharmaceutical industry.
Also Check: Define Correlational Research In Psychology
Scalable And Selective Deuteration Of Arenes
A method for the selective deuteration of anilines, indoles, phenols and heterocyclic compounds, including natural products and other bioactive molecules, has been developed. The nanostructured iron catalyst that underpins this process is prepared by combining cellulose with iron salts and has been used for the preparation of deuterated compounds on up to a kilogram scale.
Referencesisbn Links Support Nwe Through Referral Fees
- Corey, E. J., and Xue-Min Cheng. 1995. The Logic of Chemical Synthesis. New York: John Wiley. ISBN 0471509795.
- McMurry, John. 2004. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Solomons, T.W. Graham, and Craig B. Fryhle. 2004. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
- Vogel, A.I., et al. 1996. Vogel’s Textbook of Practical Organic Chemistry, 5th Edition. Prentice Hall. ISBN 0582462363.
- Zumdahl, Steven S. 2005. Chemical Principles. New York, NY: Houghton Mifflin. ISBN 0618372067.
Don’t Miss: Countdown To The Algebra 1 Eoc Answer Key
Building And Searching Reaction Networks
As we all know, one decisive character differing between humans and computers are the ability of memory. For organic chemistry experts, they often memorize hundreds of classic reactions and rules, but modern computers have the ability to store and search for chemical databases as large as the entire set of known molecules and reactions. In a computer scientists’ view, chemical reactions are sets of data indicating relationships or connections of compounds, and this kind of existence can be represented as data structures such as connections or networks. According to these ideas, Grzybowski et al. did such a kind of transformation in early 2000s and finally finished the Network of Organic Chemistry , which contains more than ten million organic reactions connecting a similar number of compounds .
Figure 1. Schematic representation of a local part of the Reaction Networks. Reactions included in this figure are: A + B = C B + C = D C = E.
Schllkopf Amino Acid Synthesis
Asymmetric synthesis of amino acids 4 from dihydropyrazines 1, 3. .
Pyrazine .3 To 1 in THF at â70 °C was added 1.8N BuLi in hexane followed after 15 min by CH2Br2 in THF . After stirring 30 h at â70 °C, workup afforded 3.2 g of 2 , bp 76â80 °C . Reaction of 2 with t-butylmercaptan in DMSO and KOtBu for 5 h at 70 °C gave after workup and distillation 0.837 g of 3 , bp 80â90 °C . Hydrolysis of 3 with dil HCl at r.t. afforded amino acid 4.
| 1 |
Robert E. Gawley, Jeffrey Aubé, in, 2012
You May Like: Jonathan Thomas Beth Thomas
Synthesis Of Commonly Used Synthetic Biocompatible Biopolymers
Chemical synthesis of polymers has been majorly carried out by following polymerization methods :
-
Addition polymerization: This polymerization involves the addition of monomers without loss of any atom or molecules. These reactions may proceed by free radical cationic or anionic mechanisms. Addition polymerization is classified into four types: Bulk, solution, emulsion, and suspension.
-
Condensation polymerization: In this polymerization, a monomer having a reactive group joins each other by releasing small molecules. Condensation polymerization is classified into three types: Melt, solution, and azeotropic dehydration.
-
Ring opening polymerization: It is also known as chain-growth polymerization. In this type of polymerization terminal end of polymer attack on cyclic monomer to form a polymer chain. It can follow cationic, anionic, radical, and coordination or insertion mechanism.
Ring opening polymerization of -caprolactone/2-methylene-1,3-dioxepane in presence of catalyst and initiator afforded PCL . Various metallic , organic and enzymatic catalytic systems have been explored for ring-opening polymerization . Out of all metals, tin octoate has been extensively used due to its high activity. Various eco-friendly enzyme-catalyzed reactions have also been reported for the PCL synthesis. Recently, ultrasonication, supercritical CO2 technologies, and microwave irradiation are also used to synthesize PCL .
Fig. 5. Various polymerizations for the synthesis of polycaprolactone.
What Is Organic Synthesis
Use this explainer to familiarise students with organic synthesis, including carbonyl chemistry, as part of their preparation for the Chemistry Olympiad
Organic synthesis is a common topic in Olympiad questions, but its often less familiar to students at the time they take part in the competition. This explainer provides an introduction to this topic, with a particular emphasis on carbonyl chemistry, to help students get to grips with the key ideas.
Students should read the introduction, before taking a look at a worked example of a past Olympiad question on the topic. They can then have a go at answering a past question themselves, before checking their answers.
You May Like: Who Is Khloe Kardashian’s Real Dad
What As A Synthesis Reaction
-
Synthesis reactions happen when two separate atoms or molecules come together to form a new molecule or compound. When a synthesis reaction occurs, much of the time, energy is released, and the reaction is exothermic. An endothermic result, on the other hand, is probable. Synthesis reactions, which involve single displacement, double displacement, and combustion reactions, are one of the most common types of chemical reactions.
-
Many synthesis reactions are much more complicated than A + B = C, as shown above. Chemical synthesis reactions, for example, may require more than two different molecules, and mixtures of ingredients, as well as unreacted starting materials, may occur. Byproducts can form as a result of the formation of intermediate molecules. Furthermore, depending on the orientation of the two colliding reactant molecules, both the desired product and byproducts can form, affecting product purity.
-
Synthesis reactions come in a variety of forms. Nucleophilic and electrophilic addition and substitution reactions, for example, are diverse reaction forms that can lead to a plethora of synthesis reactions. The composition of the final reaction mixture is determined by the conditions under which two or more reactants combine to form a more complex molecule.
Chemical Properties Of Sodium Carbonate Na2co3
1. Anhydrous sodium carbonate is stable towards heat. It melts without decomposition at 852oC.
2. Aqueous solutions of sodium carbonate are mildly alkaline due to hydrolysis which releases OH ions.
Na2CO3 + 2H2O H2CO3 + 2Na+ + 2OH
3. Aqueous solutions of sodium carbonate absorb carbon dioxide from the air forming sodium hydrogen carbonate.
Na2CO3 + H2O + CO2 2NaHCO3
4. Sodium carbonate reacts with acids like weak vegetable acids, such as lime juice liberating carbon dioxide.
Na2CO3 + 2H+ 2Na+ + H2O + CO2Na2CO3 + 2HCl 2NaCl + H2O + CO2
Read Also: Algebra 2 Parcc
Automatically Learning Reaction Rules
Manual encoding of organic reaction rules has some obvious disadvantages. Since it relies on the experience of a small number of chemists, it usually did not cover enough fraction of the reaction space and few of them can be as ample as Syntaurus. Moreover, it is not realistic to exhaustively define the full substrate scope and incompatibilities for every possible reaction, and conflicting reactivity is rarely black and white incompatibility depends on the exact nature of the reacting molecules. These factors motivate the development of an automated approach to the forward reaction evaluation.
Systems with machine-generated chemistry rules were first published in the early 1990s such as the example SYNCHEM , which also use machine learning to increase its knowledge base. The KOSP program attempts to extract rules from reaction databases by clustering reactions based on characteristics of atoms within three bonds of a disconnection site. Similarly, RETROSYN also provided an interactive search based on finding single disconnections by similarity with precedent reactions. The system ARChem Route Designer developed by SymBioSys realized a systematic mode for automatically extract reaction rules and applied these rules in retrosynthetic design. However, it also has the limitation of not accounting for stereochemistry and/or regiochemistry like most rule-based system. Figure 2 illustrates how ARChem Route Designer learns reaction rules from reaction pools.
What Is An Asymmetric Synthesis
Chemistry as a subject is spread to a vast context and does not limit itself to a few formulas and chemical names. There is actually more to it than we can imagine. As an instance of this, we are here to give you a glimpse of one such chemical phenomenon that has its role in multiple applications of human life and the environment as a whole, asymmetric synthesis.
Asymmetric synthesis, also known as enantioselective synthesis, might at first sound like a complex chemical phenomenon, but to your surprise, it isn’t. The term ‘asymmetric’ in itself tells us that something over here is out of symmetry, but what? Let’s find out!
Pertaining to the fact that this phenomenon is circumferenced by chemistry, we may understand this in simpler terms for getting a clear insight.
Each compound has multiple molecules, and every molecule has its own structural symmetry. However, when this structural molecule symmetry is disturbed, it acts to affect the compound by transforming it into lopsided segments of compounds.
As you can expect, these unbalanced proportions further differ in their asymmetrical structures at the central point, which remains the most affected.
IUPAC has a relatively complicated definition for us in store: It defines this chemical synthesis as a reaction in which one or more new elements of chirality are formed in a molecule that produces stereoisomeric products in unequal amounts.
The involvement of organic compounds is a prerequisite in most circumstances.
Read Also: Electron Vs Molecular Geometry
Examples Of Synthesis In A Sentence
synthesissynthesis BostonGlobe.comsynthesisScientific AmericanThe New York Review of Bookssynthesis Quartzsynthesis Quanta Magazinesynthesis The New Republicsynthesis Forbessynthesis New York Times
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘synthesis.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Conclusions And Future Perspectives
Chemical synthesis of silver nanoparticles where different chemicals are used as reducing, stabilizing, and capping agents is relatively well established compared to biological and physical synthesis. This is because chemicals used in silver nanoparticles synthesis offer diverse reducing, stabilizing, and capping capabilities and that, unlike biological methods, chemical synthesis can be executed over wide temperature, concentration, and pH ranges.
Reaction parameters such as temperature, pH, time, type of reducing and capping agents and their concentrations, and ratio of silver source to the reducing/capping agent have been demonstrated to have a huge influence on nucleation extent and rate, particle growth, shape, size, dispersity, and stability. Manipulation of parameters, therefore, provides an avenue through which silver can readily be adapted to a targeted application, for instance, surface-enhanced Raman spectroscopy, via selective and accurate control of properties pertinent to this application.
Silver ions ratio to citrate is also a key consideration in silver nanoparticles synthesis since citrate ions serve as both reducing and capping agents. This necessitates the use of citrate ions concentration higher than the stoichiometric value to ensure that all silver ions are reduced to zerovalent silver nanoparticles.
Jean Berthier, in, 2013
Recommended Reading: Oxygen Difluoride Polar Or Nonpolar
Importance Of Synthesis In Chemistry
The organic synthesis reactions are used greatly for the manufacturing of substances required for daily life.
Besides synthesizing essential products, the chemists also synthesize naturally-existing compounds in the lab to understand their nature and structure.
Similarly, synthesis allows chemists to form chemical compounds that don’t exist in nature to elevate the living standards and for research purposes.
The main goal of synthesis is to produce final products in larger quantities in a shorter time. Moreover, almost all kinds of chemical compounds undergo synthesis reactions.
However, organic compounds possess the highest potential to perform complex compounds by synthesis reactions. Click on to learn about balancing complex chemical equations.
What Is Synthesis In Chemistry
Let us understand how to define Synthesis Reaction in Chemistry.
The process of creating complex chemical compounds from simpler ones is known as chemical synthesis. It is the method by which many essential substances for everyday life are obtained. It can be used to make any kind of chemical compound, but organic molecules are the most common. Chemists make synthetic versions of chemical compounds found in nature to learn more about their structures. Chemists may also use synthesis to create compounds that do not exist naturally for research purposes. Synthesis is used in industry to produce large quantities of materials.
In this article, we will study about synthesis reaction, what is chemical synthesis, examples of synthesis, the meaning of synthesis in science in detail.
Read Also: Holt Mcdougal Geometry Chapter 7 Test
Route Recommendation For Cetirizine
To examine the synthetic routes designed by CompRet in detail, we have applied CompRet to cetirizine, a drug whose reported synthetic route is relatively simple . Here, the results of changing the template set size and the maximum depth md are shown, followed by the routes recommended by CompRet using three scoring methods: REF, MSCS, and STEP. We also performed additional experiments for several molecules the results are shown in Fig. S5 in Additional file . First, the construction of the chemical reaction network and the synthetic routes for different template set sizes were investigated. The value of md was fixed to six. Figure shows the time taken for the network constructions. The dotted line indicates that the first route has been found. Finding a single route for the top 100 and 500 template cases required an extended period of time, because the number of candidate routes increased exponentially with the increase in the number of templates. The blue line shows the result for size 50. In this case, the network construction was completed in approximately 30 seconds. In the cases of size 100 and 500, the number of routes increased significantly thus, enumeration was halted when the number of routes exceeded 1,500,000. In the case of size 500, the time taken to find 1,500,000 routes was less than 2,000 seconds.
Fig. 5Fig. 6Fig. 8