Why Is Sio2 Tetrahedral
In silicon dioxide, each silicon atom links to four oxygen atoms by single bonds directed toward the corners of a regular tetrahedron, and SiO4 tetrahedra share oxygen atoms. This arrangement gives a three dimensional, continuous, silicon-oxygen network. A quartz crystal is a macromolecule of silicon dioxide.
S To Follow For Drawing The Of2/f2o Lewis Structure
1. Count total valence electron present in OF2
In the first step, you need to find how many valence electrons are present in the OF2 molecule. As oxygen belongs to the 16th group in the periodic table and fluorine is in the 17th group.
Oxygen valence electrons = 6
Fluorine valence electrons = 7
Total valence electrons available for drawing the OF2 lewis structure = 6 + 7 = 20 valence electrons
2. Find the least electronegative atom in OF2
After finding total valence electrons, we need to find the least electronegative for placing them at the center of the F2O lewis structure.
As electronegativity increase from left to right in the periodic table. So, oxygen is at the left and fluorine at the right in the periodic table. Hence, oxygen is less electronegative than a fluorine atoms.
Place Oxygen at the center in the lewis diagram and fluorine spaced evenly around it.
3. Connect oxygen and fluorine with a single bond
Now in this step, we will start to draw the Lewis structure of F2O by simply connect a fluorine atom with a central atom with a single bond.
= 16 valence electrons
Now we are left with 16 more valence electrons.
4. Place remaining valence electrons starting from the outer atom first
As we have a total of 16 valence electrons more and we need to complete the octet rule of atoms in this step. Start with the outer atom first.
Place 6 electrons around each fluorine atom in the 3rd step structure.
Lets see in the next step.
What Are The Electron And Molecular Geometry Of Of2
Themolecular geometry of OF2 is bent and its electron geometry is tetrahedral because the presence of two lone pairs on the central atom creates repulsion with bonded pairs of electrons, as a result, all outer atoms pushes down in order to minimize the repulsion according to the VSEPR theory, and that makes its shape look bent.
Also, the presence of three lone on each fluorine atom repels one another that makes its bond angle somewhat wider.
Lewis dot structure of OF2 plays an important role to determine the geometry of it because it helps to find how many bond pair and lone pair electrons OF2 molecule contains.
Also Check: What Is A Dependent Variable In Math
S Used To Find The Shape Of The Molecule
To sum up there are four simple steps to apply the VSEPR theory.
S To Form Of2 Lewis Structure Diagram
Step 1: Find the Total number of Valence Electrons.
The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule.
Oxygen belongs to group 16, the chalcogen family, and has a valency of 6. Fluorine belongs to the family of halogen in group 17 and has a valency of 7.
Therefore, the total number of valence electrons = 6 + 7*2 = 20.
Step 2: Identify the Central Atom
Now, we have to decipher the central atom in this molecule. If we look at the Pauling Electronegativity chart, we can find out that the least electronegative element among oxygen and fluorine is Oxygen .
The central atom in an oxygen difluoride molecule is Oxygen.
Step 3: Sketch the Skeletal Diagram of the Molecule
In Lewis Structure, we use atomic symbols like C for carbon, H for hydrogen to represent the constituent atoms, and electron dot notation to represent the valence shell electrons.
Let us look at the below skeletal sketch:
- The atomic symbols:
- Atomic symbols along with dot notations:
Step 4:
The main group elements of the periodic table have a tendency to attain the octet configuration of the noble gas elements present in group 18 of the same period. This is known as octet fulfillment since the elements want to achieve eight electrons in their valence shells.
For example, Carbon tends to attain a Neon configuration.
Exception: Hydrogen tends to achieve Helium configuration, hence only two electrons in the outer shell.
Step 5: Formal Charge Concept
Let us see:
Also Check: Which Of The Following Perspectives Dominated American Psychology For Decades
What Is The Molecular Geometry Of Cs2
linearThe molecular geometry of CS2 is linear with symmetric electron region distribution around the central atom.
Is cs2 trigonal planar?, Answer: Since C2H2 is a linear molecule the C must be sp. Also only sp carbon can form a triple bond. sp2 carbon would give a trigonal planar arrangement.
Furthermore, What is the molecular geometry of cs2 enter the molecular geometry of the molecule?, The molecular geometry of CS2 is AX2 or linear.
Finally, What is the molecular geometry of OF2?, OF2 Molecular Geometry
As only atoms are bonded with the central atom, it has a molecular geometry similar to AX2 that corresponds to linear geometry. Although the electron geometry of OF2 is tetrahedral, its molecular geometry is linear.
Find Hybridization Number Of Of2
Now find the hybridization number of OF2 to further determine its molecular and electron geometry.
Use this Formula to find the Hybridization number.
H = N.A. + L.P.
N.A. = Number of atoms attached to the central atom
L.P. = lone pairs on that central atom
Hence as we see in an OF2 lewis structure, the lone pair on the central atom is two, and the number of atoms attached to it is also two.
Put these values in the given hybridization formula.
H = 2 + 2
= 4 is the hybridization number of the OF2 molecules.
So, the hybridization of OF2 is Sp³.
Don’t Miss: Geometry Segment Addition Postulate Worksheet
Determine The Electron Geometry Molecular Geometry And Idealized Bond Angles For Each Of The Following Molec
1- Determine the electron geometry for each molecule.
2- Determine the molecular geometry for each molecule.
3- Determine the idealized bond angle for each molecule.
4- In which cases do you expect deviations from the idealized bond angle?
a) CF4
-
2. a is tetrahedral, b is trigonal bipyramidal, c & d are bent
3. they’re all 109.5 degrees
4. every compound except CF4 because it doesn’t have any lone pairs…
Source:MC
-
This Site Might Help You.
Determine the electron geometry, molecular geometry, and idealized bond angles for each of the following molec?
1- Determine the electron geometry for each molecule.
2- Determine the molecular geometry for each molecule.
3- Determine the idealized bond angle for each molecule.
4- In which cases do you expect deviations from the idealized bond angle?
a) CF4
What Are Polar And Nonpolar Molecules
The molecules are held together by the bonds. These bonds can be metallic, covalent, ionic, and hydrogen bonds.
Among these bond forces, the ionic and covalent bonds are strongest and majorly used in the chemical compounds.A covalent bond can be polar as well as nonpolar depending upon the factors discussed below in detail.Polar molecules: these are the molecules in which the net dipole moment of the molecule comes out to be nonzero.
The covalent bond formed between two atoms is said to be polar if the electronegativity of both atoms is not equal.
These atoms unequally share the bonded pair of electrons. The more electronegative atom gains partial negative charge and other atom gets a partial positive charge.
Examples of such molecules are SO2, OF2, etc. You can check out the reason for the polarity of SO2.
Nonpolar molecules: These are the molecules in which the net dipole moment is zero. The atoms forming a nonpolar covalent bond have the same electronegativity value.
These atoms share the bonded electrons equally such that equal charge is present on both of atoms. Examples of such molecules are CO2, H2, etc.
The main factors that determine the polarity of a molecule are electronegativity, geometrical shape, and dipole moment.
Note: It is possible to have the existence of a polar bond within a nonpolar molecule. Because due to symmetrical geometry the polarity is canceled by each other.
Also Check: Define Electron Geometry
Why Is Of2 A Polar Molecule
The molecule of OF2 is polar in nature because of its bent shaped structure and difference between the electronegativity of oxygen and fluorine.
The geometrical shape of Oxygen difluoride is similar to that of water ie V-shaped bent structure.
The OF2 molecule has oxygen and fluorine atoms that have an electronegativity difference of around 0.54 units.
Being more electronegative, the fluorine atom attracts the bonded pair of electrons slightly towards it and it gains a partial negative charge and oxygen atom gains partial positive charge.
As a result, the O-F bonds ensure the dipole moment in the same direction and the net dipole moment of the whole molecule is also nonzero.
There are 2 lone pairs on oxygen atom and 3 lone pairs on both fluorine atoms. As per the VSEPR theory, the repulsion among lone pairs and bond pairs causes the distortion in shape.
Therefore, the shape os OF2 is changed to bent shape.
The asymmetric geometrical structure of a molecule makes it polar because, in these shaped molecules, the dipoles do not cancel each other. They gave resultant dipole as nonzero.
Due to these factors, the OF2 molecule is a polar molecule.
How To Draw Lewis Structure Of Ci4
CI4 lewis structure contains carbon atom at middle position whereas four iodine atoms surround to it. A total of 12 lone pairs and 4 bonded pairs are present in the lewis structure of CI4.
The lewis structure of CI4 is similar to CCl4 and CF4, since, they all are in the same group in the periodic table and contain the same number of valence electrons.
You May Like: What Is Figure Ground Perception Psychology
Is Sio2 A Sp3
Silicon dioxide has a giant molecular structure. Each silicon atom is connected to four oxygen atoms and each oxygen atom is connected to two silicon atoms, which means that sp hybridisation isnt possible for Si. Edit: Both oxygen and silicon have four electron domains and are therefore both sp3 hybridised.
Is Of2 Polar Or Nonpolar
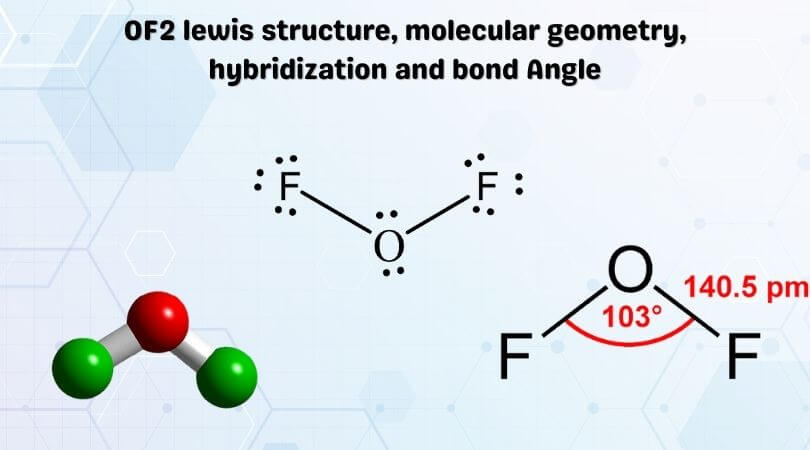
Oxygen difluoride is a chemical compound having its chemical formula as OF2. It was first reported in the year 1929. As per the VSEPR theory, the shape of the molecule is bent like that of a water molecule but have different properties. Many students might have a query about whether the OF2 molecule is polar or not. In this article, I will answer this question and will cover the surrounding topics too.
So, Is OF2 Polar or Nonpolar? OF2 is polar in nature because of its bent shaped geometrical structure and difference between the electronegativity of Oxygen and Fluorine atoms. As a result, the dipole moment of the molecule turns out to be nonzero making the OF2 a polar molecule.
Oxygen difluoride is also known by its other name hypofluorous anhydride. It is a colorless gas in appearance at room temperature. It turns out to pale yellow colored liquid on condensation.
It is a foul-smelling substance. The melting point of oxygen difluoride is around 223.8 °C or 370.8 °F.
If we talk about the chemical composition of OF2, it consists of 1 Oxygen atom and 2 fluorine atoms.
The electrons in the outermost shell of fluorine are 7 and that of oxygen is 6. The fluorine atom needs 1 electron and oxygen atom needs 2 electrons for their stabilization ie to complete their octet.
Accordingly, the two fluorine atoms form a single covalent bond with an oxygen atom. And the molecules become stable leaving behind 2 lone pairs on oxygen atom and 3 lone pairs on both fluorine atoms.
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
Factors Affecting Polarity Of A Molecule
Electronegativity: The term electronegativity is the strength of an atom to attract the bonded pair of electrons towards it. More electronegative atom attracts bonded electron pairs more towards itself.
The difference between the electronegativity of two atoms forming a covalent bond exhibit polarity in their bond.
Polarity is directly proportional to the difference between the electronegativity of the atoms.
Geometrical shape: If the shape of a molecule is symmetric, the molecule is nonpolar in nature because of the following reasons.
The symmetrically shaped molecules in which atoms have the same electronegativity have equal charge distribution on them and are nonpolar in nature.
If the symmetrical molecule has dipoles within it, then these dipoles get canceled by each other.
Therefore, symmetrically shaped molecules are nonpolar in nature.
Dipole moment: The dipole moment of a molecule is the measure of its polarity. The polarity of a molecule is directly proportional to the dipole moment of a molecule.
It is denoted by D.
Mathematically, it is the product of the charge and the distance between them.
D= Q*R
Formula Of2 Name Valence Electron Electrons Domains Oxygen H Parent Molecular Geometry Geometry Tetrahedral Bent So
Formula OF2 Name Valence Electron electrons domains Oxygen H Parent Molecular geometry geometry Tetrahedral Bent SO Hybridization bonds bonds Sp3 2 O SF. Gal Diflouride 20 Sulfur Trioxide 32 Sulfur Tetraflouride Galiumin Iodide Xenon Diflouride Arsenic Bromite Carbon Disulfide XeF: AsBrs CS2 Phosphate Chlorine Triflouride Xenon Tetraflouride Include Lewis structures and 3-D structures on a separate page. Hybridization refers to the central atom for the structure.
Recommended Reading: Ccl4 Bond Angles
Follow Some Steps For Drawing The Lewis Dot Structure For Ci4
1. Count total valence electron in CI4
First of all, determine the valence electron that is available for drawing the lewis structure of CI4 because the lewis diagram is all about the representation of valence electrons on atoms.
So, an easy way to find the valence electron of atoms in the CI4 molecule is, just to look at the periodic group of carbon and iodine atoms.
As the carbon atom belongs to the 14th group in the periodic table and iodine is situated in the 17th group, hence, the valence electron for the carbon is 4, and for the iodine atom, it is 7.
Total number of the valence electrons in carbon = 4
Total number of the valence electrons in iodine = 7
Total number of valence electrons available for the CI4 Lewis structure = 4 + 7×4 = 32 valence electrons
2. Find the least electronegative atom and place it at center
As we know, electronegativity increase as we move from left to right in the periodic table. Hence, the iodine atom is more electronegative than a carbon atom. Therefore, it can never be placed in a central position because it is less prone to share electrons.
So, place the carbon in the middle position and spread the four iodine atoms around it.
3. Connect outer atoms to central atom with a single bond
In this step, join all outer atoms to the central atom with the help of a single bond.
In, CI4 molecule, iodine is the outer atom, and carbon is the central atom. Hence, joined them.
= 24 valence electrons
Formal charge =
For carbon atom:
What Is The Molecular Geometry Of Of2 Of 2
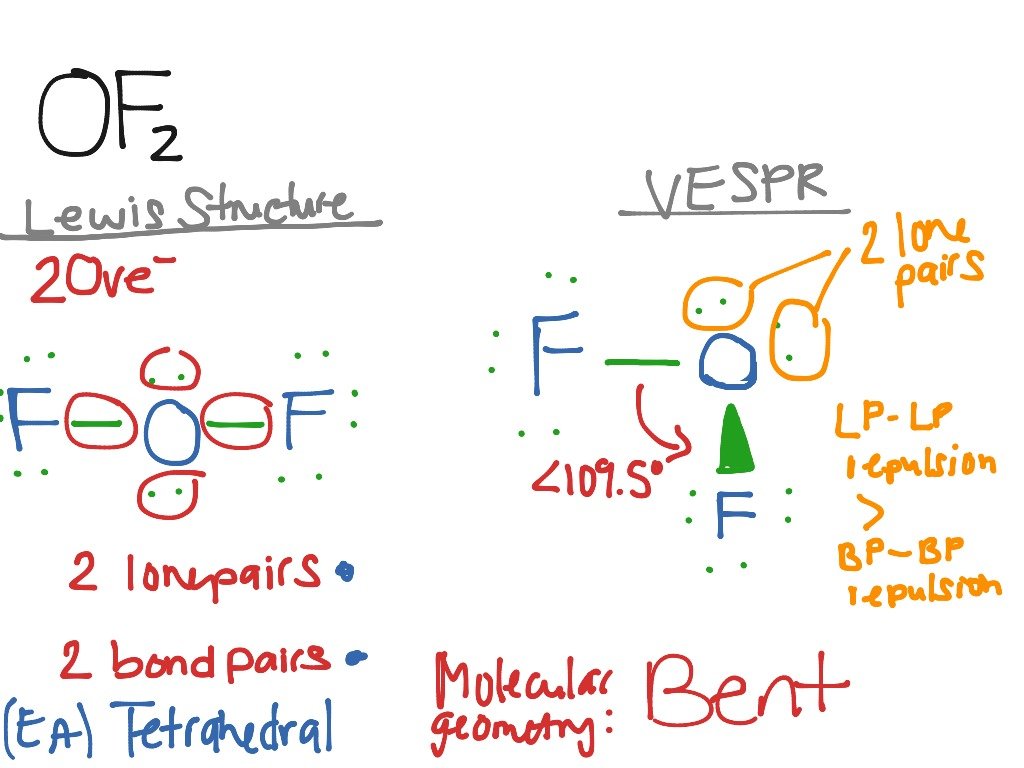
So, according to the lewis dot structure of OF2, oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons. OF2 formula becomes AX2N2. According to the VSEPR chart, the molecule which has the AX2N2 formula their molecular shape is bent and electron geometry is tetrahedral.
Don’t Miss: Geometry Segment Addition Postulate Worksheet
Oxygen Difluoride Molecular Geometry
You can use the AXN method also to determine the molecular and electron geometry of OF2.
- A represents the central atom.
- X represents the bonded pairs of electrons to the central atom.
- N represents the lone pairs of electrons on the central atom
So, according to the lewis dot structure of OF2, oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons.
OF2 formula becomes AX2N2.
According to the VSEPR chart, the molecule which has the AX2N2 formula their molecular shape is bent and electron geometry is tetrahedral.
| Bonded atoms |
| Tetrahedral |
VSEPR Chart
The bond angle of OF2 is 103º because two lone pairs on the central atom decrease the value bond angle of OF2 from its normal value.
What Is The Molecular Geometry Of Ci4
The molecular geometry of CI4 is tetrahedral because the central atom carbon is bonded with four iodine atoms and it contains no lone pair that means, it is an AX4 type molecule.
A represent central atom
X represent the number of bonded atom to central atom
According to VSEPR theory or chart, the AX4 type molecule forms tetrahedral molecular geometry or shape.
You May Like: 4.5 Practice A Geometry Answers
Predict The Molecular Shape Of The Of2 Molecule
According to VSEPR theory, OF2 geometry is linear. This is because of the repulsion between the lone pair electrons of oxygen and fluorine.
Each fluorine atom has seven valence electrons, but as there are two Fluorine atoms, we will multiply the number by 2. So we have 14 valence electrons from Fluorine atoms.
Electronic configuration of oxygen : 1s2 2s2 2p4
The 2s and 2p orbitals of the Oxygen atom are hybridized, which means there is a formation of four hybridized orbitals: 2s, 2px, 2py and 2pz. So the molecule will become sp3 hybridized.
= 6 + 7 x 2
= 20 valence electrons
- Thus, there are 20 valence electrons available for OF2 that help the atoms to form bonds.
- O undergoes sp3 hybridization. Two-hybrid orbitals contain lone pairs. Oxygen Difluoride has a similar arrangement to H2O. Although the electron geometry of OF2 is tetrahedral, its molecular geometry is linear.
Was this answer helpful?
Of2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram
Oxygen Difluoride or OF2 is a chemical compound formed by the reaction between halogen fluorine and dilute aqueous solution of NaOH .
The equation for the preparation of Oxygen Difluoride:
2F2 + 2NaOH > OF2 + 2NaF + H2O
It is a colorless gaseous compound exhibiting a strong peculiar odor and acts as an oxidizer. It has a boiling point of 128.40 K and a melting point of 49.3 K. It has a molar mass of 53.996 g/mol and a density of 1.88 g/l as a gas at room temperature.
Although it has its use as a strong oxidizing agent, for example, in propellants of rocket fuels, this property also makes the compound unsafe.
OF2 can explode on contact with water and is said to be highly corrosive to the skin. It can also affect the eyes and the cardiovascular system.
Let us have a quick but detailed look into the inside of the molecule and discuss its nature of chemical bonding.
Read Also: Founder Of Behavioral Psychology