Shielding Is The Measure O The Effect Of Inner Sub Shells Of The S P D And F On Their Interference Of The Nuclear Charge Of The Protons On The Valence Electron
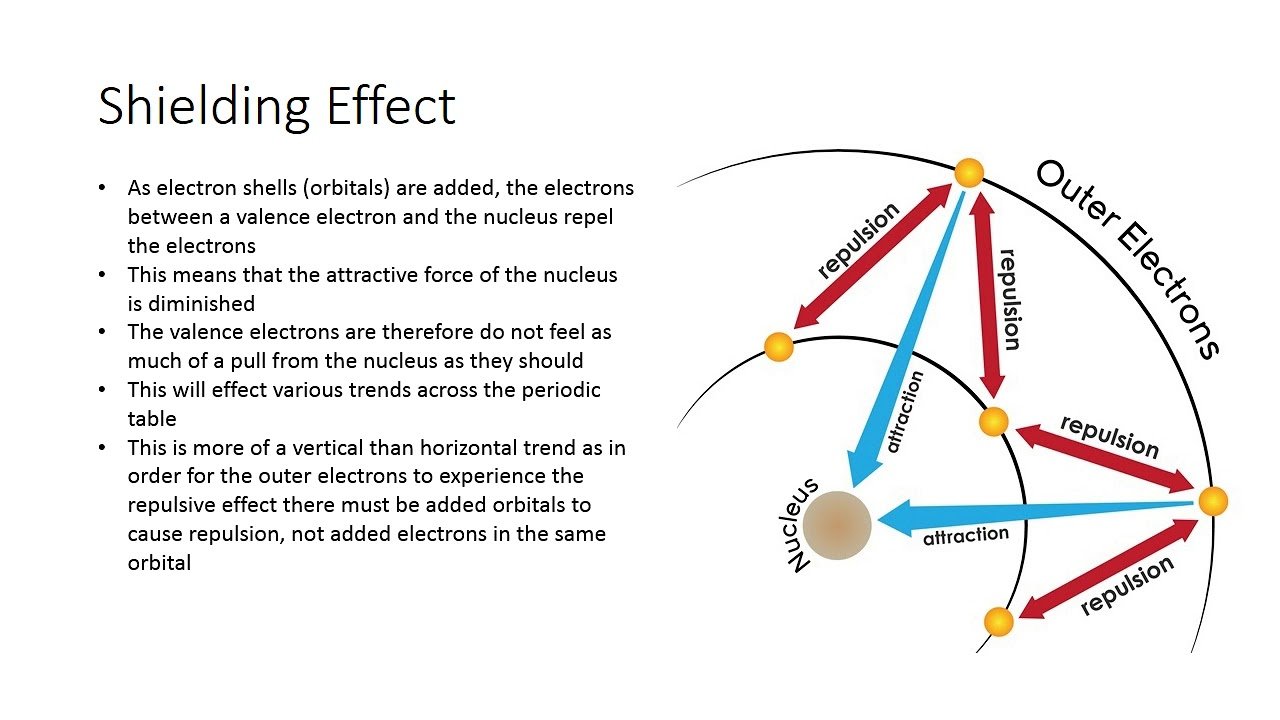
As well as effective nuclear charge and its effects on the valence electrons we also need to consider shielding very often when looking at chemical properties of elements and trends and patterns in the periodic table. The number of protons in the nucleus is always shielded by the inner shells, weakening the attraction of the nucleus to the valence electron. Shielding can also causes repulsion between the sub orbitals pushing electrons further out as the orbitals overlap and cause repulsion between each other.
For example looking at elements in the same group if we look at sodium and rubidium we can see that the sodium nucleus has 2 shells of inner electrons between the nucleus and the valence electron meaning it has 2 shells of electrons that interfere with the attraction between the nucleus and the outer electron. Where as rubidium has 4 shells of electrons between its nucleus and the valence electron, which despite having a larger nucleus and more protons has a larger number of shells reducing the effect of the nucleuses attraction to the outer electron.
For example an s shell will shield a p shell because it is closer to the nucleus and has a greater attraction to the nucleus, this weakens the attraction of the electrons in the p sub shell. So instead of experiencing the nuclear charge the p shell experiences the effective nuclear charge. We can calculate this by subtracting the atomic number from the Shielding constant S.
Zeff = Z-S
The Shielding Effect And Effective Nuclear Charge
- Calculate effective nuclear charges experienced by valence electrons.
Key Points
- The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons.
- The shielding effect explains why valence-shell electrons are more easily removed from the atom. The effect also explains atomic size. The more shielding, the further the valence shell can spread out and the bigger atoms will be.
- The effective nuclear charge is the net positive charge experienced by valence electrons. It can be approximated by the equation: Zeff = Z S, where Z is the atomic number and S is the number of shielding electrons.
Terms
- effective nuclear chargeThat experienced by an electron in a multi-electron atom, typically less for electrons that are shielded by core electrons.
- nucleusThe positively charged central part of an atom, made up of protons and neutrons.
- core electronsThose that are not part of the valence shell and as such, are not involved in bonding.
- valence shell electron pair repulsion theoryA set of rules used to predict the shape of individual molecules.
- cationA positively charged ion, as opposed to an anion.
- valence shellThe outermost shell of electrons in an atom these electrons take part in bonding with other atoms.
- anionA negatively charged ion, as opposed to a cation.
How Do Electrons Fill In Orbitals
Electrons fill orbitals beginning on the lowest accessible power state earlier than filling increased states. Aufbau process: Decide variety of electrons for the atom of curiosity. Fill accessible orbitals beginning with the lowest-energy ranges first and keep away from pairing electrons in a single orbital till it is vital.
Recommended Reading: Grade 6 Fsa Warm Ups Answer Key
Are You Eligible For A Free Nhs Flu Vaccination
You may be entitled to a free NHS flu vaccination from your GP or local pharmacist. Find out if you are eligible today.
- Stay at home for at least 12 weeks from mid March.
- You should not leave the house for shopping or leisure, including going for a walk.
- Wash your hands regularly for at least 20 seconds.
- If you live with someone who is shielding with you, you can have contact with them inside the house as long as they do not have any symptoms.
- If you live with other people who leave the house at all, you should:
- Minimise the time you spend with them.
- Keep at least 3 steps from them.
- Avoid using the kitchen when they are there and keep your own separate crockery and cutlery.
- Have your meals in your room where possible.
- Use separate towels and if possible a separate bathroom from them.
- If you have carers coming in to provide essential services, they can continue to do so. However, they will need to practise strict hygiene.
Why Does Lithium Have Such A Low Ionization Energy
But Li has a configuration 1s2 , 2s1 i.e it has one electron in its outermost shell so get stable it needs to remove that one electron and thats why its first ionization energy is less, now Li+ is a stable atom and to remove an electron from the stable we need high energy and therefore its second ionization energy is
Also Check: Segment And Angle Addition Worksheet
Matt Hancock Vs The Sunday Times
This confusion was made apparent on 3 May, by the response that the health secretary, Matt Hancock, gave to the front page story in The Sunday Times.
The story said, correctly, that all those aged 70 and over, regardless of health conditions, have been classified as clinically vulnerable.
But it then incorrectly said that the clinically vulnerable have been asked to stay inside for at least 12 weeks, when this is actually the shielding advice given to the clinically extremely vulnerable. The piece has since been corrected.
Responding on , Mr Hancock described the story as factually wrong and misleading but made a mistake in his attempt to correct the article.
He said correctly that over 70s have not been asked to stay in lockdown for 12 weeks, but then incorrectly said that over 70s are not clinically vulnerable which they are.
The government advice on social distancing at the time, said people aged 70 or older are classed as clinically vulnerable, regardless of any medical conditions. It said these people are at higher risk of severe illness and should take particular care to minimise contact with others outside your household.
This is still the advice as of 4 June.
The NHS also lists people aged 70 or over as being clinically vulnerable, recommending they only leave home if it is essential.
But what do these two labels mean in practice?
What Influences Atomic Radius
The bigger the atomic number, the larger the atoms radius. This is especially true as you move straight down a given column on the periodic table the radius of each successive neighboring atom increases. The growing size is due to the increasing number of filled electron shells as you move down the periodic table.
Recommended Reading: Find The Length Indicated Geometry Worksheet Answers
How Does The Shielding Effect Affect Trends
earth metals more reactive as you move down
More shielding halogens more reactive as you move up
Explanation:
The shielding effect is the electrons between the nucleus and the valence electrons acting as a “shield” – repelling the outer electrons because they have the same charge, lowering the effective nuclear charge.
You can calculate the effective nuclear charge by
#Z_ = Z – S#
is the atomic number and #S# is the shielding electrons.
The more shielding electrons you have, the lower the ENC, so the less force there is holding onto the outer shell electrons.
If there is less force holding onto valence electrons, then they will be lost more easily, and likewise not gained as easily. Therefore when you move down the left-hand-side of the periodic table, atoms become more reactive – more liable to lost electrons. As you move down the right, however, atoms cannot gain electrons as easily, so as you move down the column of halogens, reactivity decreases.
This is because the left of the table lose electrons to form positive ions, while the right gains electrons to form negative ions.
Also, the lower the ENC due to greater shielding effect, the less electronegative an element will be, so whatever electrons it does have will not be pulled towards it so easily.
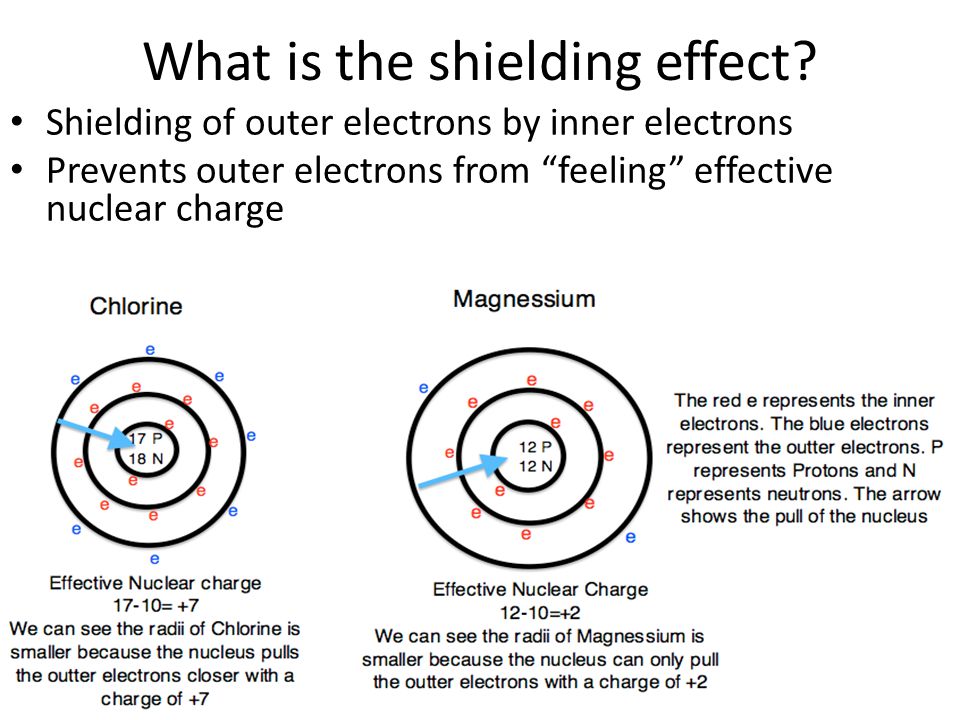
What Is The Shielding Effect
In chemistry, the shielding effect is the weakening of the attraction between an electron and an atomic nucleus with more than one electron shell. The effect is also called screening or atomic shielding.
In atoms and ions with only one electron, the total force experienced by an orbiting electron is equal to the electromagnetic attractive force that the nucleus exerts on this electron. When there are more electrons orbiting the nucleus, each electron experiences this nuclear electromagnetic attraction in addition to repulsion forces by the surrounding electrons. The magnitude of this repulsive force depends on the number of electrons, so as the number of filled electron shells increases, the net force on the outermost electrons decreases. These outer shell electrons are not as strongly bonded to the nucleus as the electrons in inner shells, explaining why valence-shell electrons are more easily removed from an atom than inner shell ones.
A larger number of orbiting electrons results in more complex repulsive interactions between these electrons, making the quantitative assessment of the repulsive force resulting from the shielding effect difficult. Techniques to determine the shielding effect include numerical solutions of the Schrodinger wave equation, using Slater empirical formulas or inferring the effect using the Rutherford backscattering spectrometry.
Recommended Reading: Fsa Algebra 1 Eoc Practice Test Answers
What Is The Goal Of A Roller Derby Game
Roller derby is a popular sport, although it is unfamiliar to many people. The basic purpose is to set one team member past the opposing team to score points. Other members of the team serve as blockers to prevent the opposing team from stopping the jammer. Blockers interfere with the interaction between the jammer and the opponents by getting between the jammer and the skaters trying to stop her.
The attraction between an electron and the nucleus of the atom is not a simple issue. Only with hydrogen is there a one-to-one relationship that can be discussed in terms of direct charge attraction. As the size of the atom increases, the number of protons and electrons also increase. These changes influence how the nucleus attracts electrons.
In general, the ionization energy of an atom will increase as we move from left to right across the periodic table. There are several exceptions to the general increase in ionization energy across a period. The elements of Group 13 have lower ionization energies than the elements of Group 2 . This is an illustration of a concept called electron shielding . Outer electrons are partially shielded from the attractive force of the protons in the nucleus by inner electrons.
Figure 1. The shielding effect is shown by the interior electron cloud shielding the outer electron of interest from the full attractive force of the nucleus. A larger shielding effect results in a decrease in ionization energy.
Table Of Effective Nuclear Charges Of The Atoms
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear charge may be approximated by the equation:
Zeff= Z – S
Where Z is the atomic number and S is the number of shielding electrons.Higher energy electrons can have other lower energy electrons between the electron and the nucleus, effectively lowering the positive charge experienced by the high energy electron.
The shielding effect is the name given to the balance between the attraction between valence electrons and protons and the repulsion between valence and inner electrons. The shielding effect explains the trend in atomic size on the periodic table and also why valence electrons are readily removed from an atom.
Read Also: What Does Abiotic Mean In Biology
Why Is Shielding Effect S More
The more shielding, the further the valence shell can spread out and the bigger atoms will be. The effective nuclear charge is the net positive charge experienced by valence electrons. It can be approximated by the equation: Zeff = Z S, where Z is the atomic number and S is the number of shielding electrons.
Strict Social Distancing For The Clinically Vulnerable
Clinically vulnerable describes the wider group of people who have been identified as being more at risk from the new coronavirus but not so severely that they need to shield themselves.
The government doesnt actually recommend that clinically vulnerable people follow any rules that differ from the universal social distancing guidance.
But these people are being told to follow that guidance carefully, and to take particular care to minimise contact with others outside of their household.
As discussed above, this group includes anyone aged 70 or older regardless of their medical conditions.
It also includes anyone who is under 70 but has health conditions including chronic mild to moderate respiratory disease, chronic heart, kidney or liver disease, chronic neurological conditions, diabetes or a weakened immune system because of conditions such as HIV and AIDS or medicines like steroid tablets.
This group also includes pregnant women and anyone classed as seriously overweight, with a body mass index of 40 or above.
This article was updated to include new government guidance on shielding.
Also Check: Eoc Algebra 1 Practice Test With Answers 2015
What Is Shielding And Who Needs To Do It
People deemed most at risk of becoming seriously ill from the new coronavirus have been advised to shield by the government, meaning they should not leave their homes and should minimise all face-to-face contact until at least the end of June.
However, there has been some confusion about who exactly is required to shield and what this means in practice. New guidance that applies from 1 June permits people who are shielding to go outside in certain situations. Our readers have asked us to explain this.
What Does 1s 2s 2p Imply
The superscript is the variety of electrons within the stage. The quantity in entrance of the power stage signifies relative power. For instance, 1s is decrease power than 2s, which in flip is decrease power than 2p. The quantity in entrance of the power stage additionally signifies its distance from the nucleus.
Read Also: Geometry Segment Addition Postulate Worksheet
Calculating The Effective Nuclear Charge:
An estimate of effective nuclear charge can be obtained from Zeff = Z – S, where Zeff = effective nuclear charge, Z = atomic number, and, S = the screening constant.””Consider aluminum: 3s23p1 “”Z = 13 S = 10 Zeff = Z – S = 13 – 10 = 3+
Dont forget that Zeff is only an estimate. Actual shielding effect is always greater that the screening constant S because core electrons are much closer to the nucleus than are valence electrons.
What Number Of Electrons Are In Every Shell
Every shell can comprise solely a hard and fast variety of electrons: The primary shell can maintain as much as two electrons, the second shell can maintain as much as eight electrons, the third shell can maintain as much as 18 and so forth. The overall components is that the nth shell can in precept maintain as much as 2 electrons.
Read Also: Paris Jackson’s Biological Parents
Which Orbitals Have The Best Power
The power of an electron versus its orbital Inside a given principal power stage, electrons in p orbitals are at all times extra energetic than these in s orbitals, these in d orbitals are at all times extra energetic than these in p orbitals, and electrons in f orbitals are at all times extra energetic than these in d ortitals.
Periodic Trends Due To Penetration And Shielding
- Effective Nuclear Charge ): The effective nuclear charge increases from left to right and increases from top to bottom on the periodic table.
- Atomic Radius: The atomic radius decreases from left to right, and increases from top to bottom.
- Ionization Energies: The ionization energies increase from left to right, and decrease from top to bottom.
- Electronegativity: The electronegativity of the elements is highest near flourine. In general, it increases from left to right and decreases from top to bottom.
Read Also: What Is Figure Ground Perception Psychology
Help From Nhs Volunteers
Many people who have to stay home for their own protection can now access help from the 750,000 NHS volunteers who have signed up to help. The services NHS volunteers can offer include:
- Collecting and delivering shopping and other essential supplies.
- Delivering medicines from pharmacies.
- Bringing them home from hospital.
- Making regular phone calls to check on people isolating at home.
- Transporting medical supplies and equipment for the NHS.
People who are eligible for help include:
- Anyone who has been advised to self-isolate and shield themselves.
- Over-70s who have underlying health conditions such as heart disease, diabetes, COPD, liver or kidney problems, or nervous system conditions such as Parkinsons disease or multiple sclerosis.
- People who are self-isolating who GPs consider especially vulnerable.
If you think you may be eligible, please contact your GP practice . They can refer you to this service.
What Is Shielding Effect
Shielding effect is the reduction in the effective nuclear charge on the electron cloud, due to differences in the attraction forces between electrons and the nucleus. This term describes the attraction forces between electrons and nucleus of an atom having more than one electron. It is also called atomic shielding.
The shielding effect gives the reduction of attraction between the atomic nucleus and the outermost electrons in an atom containing many electrons. The effective nuclear charge is the net positive charge experienced by the electrons in the outermost electron shells of an atom . When there are many inner shell electrons present, the atomic nucleus has less attraction from the atomic nucleus. That is because the atomic nucleus is shielded by the electrons. Higher the number of inner electrons, greater the shielding effect. The order of increasing the shielding effect is as follows.
S orbital> p orbital> d orbital> f orbital
There are periodic trends of shielding effect. A hydrogen atom is the smallest atom in which one electron is present. There are no shielding electrons, therefore the effective nuclear charge on this electron is not reduced. Hence, there is no shielding effect. But when moving across a period in the periodic table, the number of electrons present in the atom increases. Then the shielding effect is also increased.
Figure 01: The Shielding Effect on an Electron
Don’t Miss: Kendall Hunt Geometry Answers