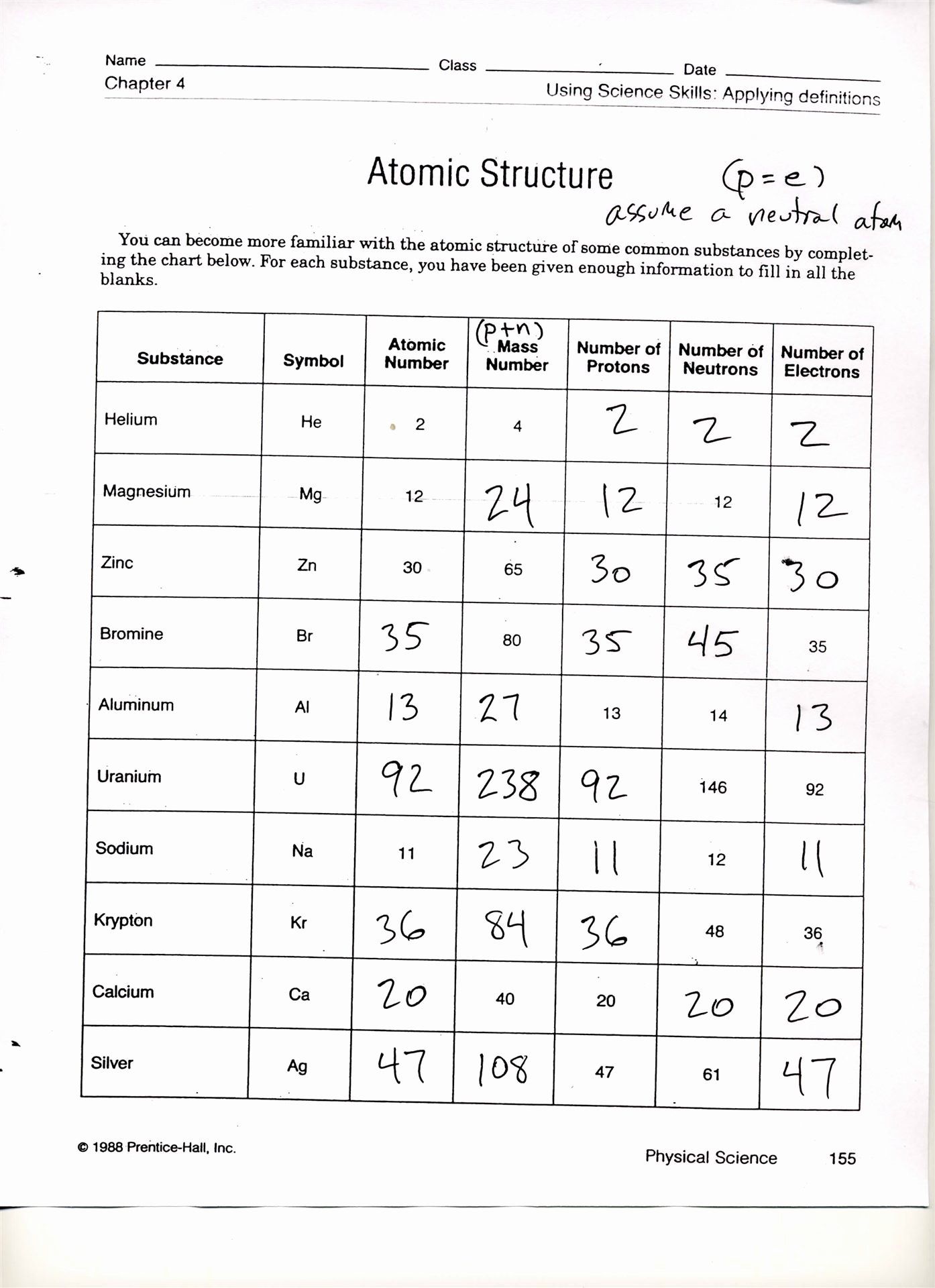
What Is A Blank Solution
we_objectID=299& rb_select_l=B& rb_search=& PHPSESSID=363d
Glossary —- blank solution
1. a solution that does not contain a detectable amount of theanalyte of interest. The
blank solution is typically used for calibration purposes.Depending on its purpose the
following blank solutions can be defined:
Calibration blank (the solution used for creating the zeroconcentration point of the
calibration graph this solution contains only the diluent usedfor making the standard
solution)
2. Reagent blank (a blank solution that contains the reagentsused to dissolve the
samples such as acids used for digestion the reading for thissolution is typically
substracted from sample readings)
3. Method blank (a blank solution that has been handled similarto a sample, and to
which the same reagents have been added, that had contact to thesame type of vessels
and that was treated by a similar procedure. This solution thanis handled to monitor
any type of contamination taking place with the method used.
The correct treatment of the analytical result for the blanksolution does have a
significant effect on the correctness of analytical results,especially for samples having
analyte concentrations close to the limit of quantitation.Especially the measurement of
the reagent and method blank must be well differentiated fromthe baseline correction.
Ice Tables: A Useful Tool For Solving Equilibrium Problems
ICE tables are very helpful tools for understanding equilibrium and for calculating the pH of a buffer solution. They consist of using the initial concentrations of reactants and products, the change they undergo during the reaction, and their equilibrium concentrations. Consider, for example, the following problem:
Calculate the pH of a buffer solution that initially consists of 0.0500 M NH3 and 0.0350 M NH4+. . The equation for the reaction is as follows:
\text_4^+ \rightleftharpoons \text^+ + \text_3
We know that initially there is 0.0350 M NH4+ and 0.0500 M NH3. Before the reaction occurs, no H+ is present so it starts at 0.
ICE table initial: ICE table for the buffer solution of NH4+ and NH3 with the starting concentrations.
During the reaction, the NH4+ will dissociate into H+ and NH3. Because the reaction has a 1:1 stoichiometry, the amount that NH4+ loses is equal to the amounts that H+ and NH3 will gain. This change is represented by the letter x in the following table.
ICE table change: Describes the change in concentration that occurs during the reaction.
Therefore the equilibrium concentrations will look like this:
ICE table equilibrium: Describes the final concentration of the reactants and products at equilibrium.
Apply the equilibrium values to the expression for Ka.
} = \frac = \frac )} }
Assuming x is negligible compared to 0.0500 and 0.0350 the equation is reduced to:
} = \frac = \frac }
Solving for x :
x = = 3.92 x 10-10
pH = -log
pH = 9.41
Preparing A Buffer Solution
There are a couple of ways to prepare a buffer solution of a specific pH. In the first method, prepare a solution with an acid and its conjugate base by dissolving the acid form of the buffer in about 60% of the volume of water required to obtain the final solution volume. Then, measure the pH of the solution using a pH probe. The pH can be adjusted up to the desired value using a strong base like NaOH. If the buffer is made with a base and its conjugate acid, the pH can be adjusted using a strong acid like HCl. Once the pH is correct, dilute the solution to the final desired volume.
pH probe: The probe can be inserted into a solution to measure the pH . Probes need to be regularly calibrated with solutions of known pH to be accurate.
Alternatively, you can prepare solutions of both the acid form and base form of the solution. Both solutions must contain the same buffer concentration as the concentration of the buffer in the final solution. To get the final buffer, add one solution to the other while monitoring the pH.
In a third method, you can determine the exact amount of acid and conjugate base needed to make a buffer of a certain pH, using the Henderson-Hasselbach equation:
\text=\text }_ }+\text
where pH is the concentration of , pKa is the acid dissociation constant, and and are concentrations of the conjugate base and starting acid.
Read Also: Geometry Lesson 1.7 Answers
What Is Blank In Analytical Chemistry
A blank or blank determination is an analysis of a sample without the analyte or attribute, or an analysis without a sample, i.e. going through all steps of the procedure with the reagents only. The latter type is the most common as samples without the analyte or attribute are often not available or do not exist.
Calculating The Ph Of A Base
The pH of bases is usually calculated using the OH concentration to find the pOH first. This is done because the H+ concentration is not a part of the reaction, while the OH concentration is. The formula for pOH is:
\text=-\text
}_ }\times }_ }=\frac \times \frac
}_ }\times }_ }== }_ }
+=\text
}_ }+ }_ }=\text }_ }=14.00
The pH can be calculated using the formula:
=-\text }
Weak bases exist in chemical equilibrium much in the same way as weak acids do. A base dissociation constant indicates the strength of the base. For example, when ammonia is put in water, the following equilibrium is set up:
\text }_+_2\text}\rightleftharpoons \text }_^ +^-}
}_ }=\frac
Bases that have a large Kb will ionize more completely, meaning they are stronger bases. NaOH is a stronger base than 2NH which is a stronger base than NH3 . As the bases get weaker, the Kb values get smaller.
Example:
Calculate the pH of a buffer solution consisting of 0.051 M NH3 and 0.037 M NH4+. The Kb for NH3 = 1.8 x 10-5.
\text }_+_2\text}\rightleftharpoons \text }_^ +^-}
}_ }=\frac
Assuming the change is negligible to 0.051 M and 0.037 M solutions:
}_ }=\frac
1.8 x 10-5=\frac
x = = 2.48 x 10-5
pOH = 4.61
Read Also: Why Do I Hate My Birthday Psychology
Why Is Distilled Water Used As A Blank In Spectrophotometry
So, to zero out the absorbance of compounds other than the analyte being determined, distilled water is used as a blank. This is because absorbance if any from the solvent, ethanol must be zeroed out as when the measurement of the actual unknown is being made, the absorbance of the solvent does not interfere.
Why Do We Use Negative Controls
On the other hand, a negative control is an experiment in which the microbiologist knows that there will be a negative outcome. This helps the analyst compare the result to a new experiment against an already results that are already known. Negative controls are always used during microbiology testing.
Don’t Miss: Geometry Dash Demon Key Hack
Solvent Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A solvent is the component of a solution that is present in the greatest amount. It is the substance in which the solute is dissolved. Usually, a solvent is a liquid. However, it can be a gas, solid, or supercritical fluid. The amount of solvent required to dissolve a solute depends on temperature and the presence of other substances in a sample. The word “solvent” comes from the Latin solv, which means to loosen or untie.
What Is The Purpose Of A Blank Cuvette
A spectrophotometer is an instrument used for detecting the presence of any light-absorbing particles dissolved in a solution and for measuring the concentration of those particles. A light source inside the spectrophotometer emits a full spectrum of white light towards a compartment where a sample liquid is placed.
Also Check: Algebra 2 Eoc Fsa Practice Test
Why Do We Need A Negative Control
On the other hand, a negative control is an experiment in which the microbiologist knows that there will be a negative outcome. This helps the analyst compare the result to a new experiment against an already results that are already known. Negative controls are always used during microbiology testing.
Error Due To The Regression Line
The “fitting” of the calibration graph is necessary because the actual response points yi, composing the line usually do not fall exactly on the line. Hence, random errors are implied. This is expressed by an uncertainty about the slope and intercept b and a defining the graph. A discussion of this uncertainty is given. It was explained there that the error is expressed by sy, the “standard error of the y-estimate” (see Eq. 6.23, a parameter automatically calculated by most regression computer programs.
This uncertainty about the -values is transferred to the corresponding concentrations of the unknowns on the x-axis by the calculation using Eq. and can be expressed by the standard deviation of the obtained x-value. The exact calculation is rather complex but a workable approximation can be calculated with:
For each value of the standards x the corresponding y is calculated with Eq. :
Then, sy is calculated using Eq. or by computer:
Then, using Eq. :
Now, the confidence limits of the found results xf can be calculated with Eq. :
xf ± t.sx
For a two-sided interval and 95% confidence: ttab = 2.78 . Hence all results in this example can be expressed as:
Xf ± 0.08 mg/L
Thus, for instance, the result of a reading y = 0.22 and using Eq. to calculate xf = 0.29, can be reported as 0.29 ± 0.08 mg/L.
x±2s
Don’t Miss: Who Are Paris Jackson’s Biological Parents
What Is Blank Control
Blank Control This is when only the background solutions are tested, for example, only the buffer with no sample and no treatment. This can be useful to calibrate any machines used to measure the target or analyte, or after the test, as a background control for all tests, negative control, positive control and samples.
What Does Ph Mean In A Buffer

In chemistry, pH is a measure of the hydrogen ion concentration in a solution. The pH of a buffer can be calculated from the concentrations of the various components of the reaction. The balanced equation for a buffer is:
\text \rightleftharpoons \text^+ + \text^-
The strength of a weak acid is usually represented as an equilibrium constant. The acid-dissociation equilibrium constant , which measures the propensity of an acid to dissociate, for the reaction is:
\text_} = \frac
The greater x is than , the greater the value of Ka, the more the formation of H+ is favored, and the lower the pH of the solution.
Read Also: Geometry Segment Addition Postulate Worksheet
How Do You Make A Blank Solution
Blank Solutions
Why Can We Not Use Water Instead Of Blank Control Solution In Enzyme Catalysis With Peroxidase Experiment
I have to do a lab about enzyme catalysis with peroxidase in my chemistry class. We are to use the blank control solution in the experiment. My question is, why can we not use water instead? I did some research about this and someone says something like “It is because the water blank absorbs so much light that the usable scale is compressed, thus, causing a poor accuracy.” Is this the exact reason why we do not use water in this experiment?
It is because the water blank absorbs so much light that the usable scale is compressed, thus, causing a poor accuracy.
For water, the absorption coefficients $\alpha\ $ at $\lambda$ = 680, 580, 535, 480 and 380 nm are 0.47, 0.09, 0.045, 0.013 and 0.011.
That is not a lot.
On the other hand, you never know about impurities in your buffers or other components .
Therefore, you usually do not measure against “just the solvent”, but against the solution of “everything except the probe” to record the complete background.
You May Like: Biotic And Abiotic Meaning
Why Is Water A Blank Solution
So, to zero out the absorbance of compounds other than the analyte being determined, distilled water is used as a blank. This is because absorbance if any from the solvent, ethanol must be zeroed out as when the measurement of the actual unknown is being made, the absorbance of the solvent does not interfere.
What Is The Purpose Of The Blank Solution In A Beer’s Law Experiment
purposeblank
. Then, what is the purpose of the blank solution in this experiment?
The ‘blank‘ allows you to set the spectrophotometer to zero before you measure your ‘unknown’ solution. The ‘blank‘ solution will contain everything that the ‘unknown’ solution except for the think you wish to measure.
Also, what is the purpose of doing a Beer’s Law plot? The law states that the concentration of a chemical is directly proportional to the absorbance of a solution. The relation may be used to determine the concentration of a chemical species in a solution using a colorimeter or spectrophotometer. The relation is most often used in UV-visible absorption spectroscopy.
Also know, what is the purpose of a reagent blank in spectrophotometry?
A reagent blank is a test sample that has undergone all the same treatment as your challenge sample but without the addition of a test sample. It is to verify that any positive response to your test sample is a result of the test sample itself and not any testing conditions including contaminated solvents or glassware.
What should the blank for the colorimeter consist of?
A colorimeter is a relatively simple scientific device consisting of a light source, sample holder , light intensity sensor and means of controlling the light source and integrating transmitted light intensity.
Also Check: How To Calculate Ihd Organic Chemistry
Why Is A Blank Titration Needed
A titration is a chemical technique that is used to determine the precise amount of a substance in solution. The process is carried out by slowly adding a solution of reagent with known concentration to a solution with a different reagent of unknown concentration until the reaction between the two reagents is complete. The point at which the amounts of the reactants are equal is known as the equivalence point and is normally indicated, with the help of a chemical indicator, by a color change at the end point, which approximates equivalence.
Solution Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A solution is a homogeneous mixture of two or more substances. A solution may exist in any phase.
A solution consists of a solute and a solvent. The solute is the substance that is dissolved in the solvent. The amount of solute that can be dissolved in solvent is called its solubility. For example, in a saline solution, salt is the solute dissolved in water as the solvent.
For solutions with components in the same phase, the substances present in lower concentration are solutes, while the substance present in highest abundance is the solvent. Using air as an example, oxygen and carbon dioxide gases are solutes, while nitrogen gas is the solvent.
Recommended Reading: Who Are Paris Jackson’s Parents
Drafting An Analytical Procedure
For drafting an analytical procedure the general instructions for drafting SOPs as given in Chapter 2 apply. An example of an analytical procedure as it can be written in the form of a SOP is METH 006. A laboratory manual of procedures, the “cookery book”, can be made by simply collecting the SOPs for all procedures in a ring binder. Because analytical procedures, more than any other type of SOP, directly determine the product of a laboratory, some specific aspects relating to them are discussed here.
As was outlined in Chapter 2, instructions in SOPs should be written in such a way that no misunderstanding or ambiguity exists as to the execution of the procedure. Thus, much of the responsibility lies with the author of the procedure. Even if the author and user are one and the same person, which should normally be the case , such misunderstanding may be propagated since the author usually draws on the literature or documents written by someone else. Therefore, although instructions should be as brief as possible, they should at the same time be as extensive as necessary.
As an example we take the weighing of a sample, a common instruction in many analytical procedures. Such an instruction could read:
1. Weigh 5.0 g of sample into a 250 ml bottle.2. Add 100 ml of extracting solution and close bottle.3. Shake overnight.4. Etc., etc.
What Is A Blank Test
Blank testtesttest
. Similarly one may ask, what is a blank test in chemistry?
A blank or blank determination is an analysis of a sample without the analyte or attribute, or an analysis without a sample, i.e. going through all steps of the procedure with the reagents only.
what is a trip blank sample? Processed accurately, a trip blank ensures that your primary samples were not contaminated during sampling and transport. A trip blank is a sample of analyte-free media collected in the same type of container used for the analytical test.
Also know, what is a blank control?
Blank ControlThis is when only the background solutions are tested, for example, only the buffer with no sample and no treatment. This can be useful to calibrate any machines used to measure the target or analyte, or after the test, as a background control for all tests, negative control, positive control and samples.
What is blank in clinical chemistry?
Blank A blank solution is a solution containing little to no analyte of interest, usually used to calibrate instruments such as a colorimeter.
Also Check: Exponent Rules Worksheet 8th Grade
What Is A Mixture
Mixtures are substances that consist of two or more types of matter. Air, soil, blood, etc. are different examples of mixtures. Based on the nature of the components and their distribution, mixtures are classified as homogeneous and heterogeneous mixtures.
- A mixture that has its components uniformly distributed is known as a homogeneous mixture.
- While if the distribution is non-uniform, the mixture is called a heterogeneous mixture.
A solution is a homogeneous mixture of two or more components. Lets learn more about solutions, its properties, how to find a concentration of solutions.