Mass Number And Isotopic Mass
The mass number gives an estimate of the isotopic mass measured in atomic mass units . For 12C, the isotopic mass is exactly 12, since the atomic mass unit is defined as 1/12 of the mass of 12C. For other isotopes, the isotopic mass is usually within 0.1 u of the mass number. For example, 35Cl has a mass number of 35 and an isotopic mass of 34.96885. The difference of the actual isotopic mass minus the mass number of an atom is known as the mass excess, which for 35Cl is 0.03115. Mass excess should not be confused with mass defect which is the difference between the mass of an atom and its constituent particles .
There are two reasons for mass excess:
E/z Will Work Even When Cis/trans Fails
In simple cases, such as 2-butene, Z corresponds to cis and E to trans. However, that is not a rule. This section and the following one illustrate some idiosyncrasies that happen when you try to compare the two systems. The real advantage of the E/Z system is that it will always work. In contrast, the cis/trans system breaks down with many ambiguous cases.
Example 7.5.2
The following figure shows two isomers of an alkene with four different groups on the double bond, 1-bromo-2-chloro-2-fluoro-1-iodoethene.
| -1-bromo-2-chloro-2-fluoro-1-iodoethene | -1-bromo-2-chloro-2-fluoro-1-iodoethene |
It should be apparent that the two structures shown are distinct chemicals. However, it is impossible to name them as cis or trans. On the other hand, the E/Z system works fine… Consider the left hand structure. On C1 , the two atoms attached to the double bond are Br and I. By the CIP priority rules, I is higher priority than Br . Now look at C2. The atoms are Cl and F, with Cl being higher priority. We see that the higher priority group is “down” at C1 and “down” at C2. Since the two priority groups are both on the same side of the double bond , they are zusammen = together. Therefore, this is the isomer. Similarly, the right hand structure is .
What Are The Branches Of Chemistry And Their Definition
Ernest Z. Jackie Shlecter Priya mrpauller.weebly.com
The five major branches of chemistry are organic, inorganic, analytical, physical, and biochemistry. These divide into many sub-branches.
Explanation:
ORGANIC CHEMISTRY
Organic chemistry involves the study of the structure, properties, and preparation of chemical compounds that consist primarily of carbon and hydrogen.
Organic chemistry overlaps with many areas including
- Medicinal chemistry the design, development, and synthesis of medicinal drugs. It overlaps with pharmacology .
- Organometallic chemistry the study of chemical compounds containing bonds between carbon and a metal.
- Polymer chemistry the study of the chemistry of polymers.
- Physical organic chemistry the study of the interrelationships between structure and reactivity in organic molecules.
- Stereochemistry the study of the spatial arrangements of atoms in molecules and their effects on the chemical and physical properties of substances.
INORGANIC CHEMISTRY
Inorganic chemistry is the study of the properties and behaviour of inorganic compounds.
It covers all chemical compounds except organic compounds.
Inorganic chemists study things such as crystal structures, minerals, metals, catalysts, and most elements in the Periodic Table.
Branches of inorganic chemistry include:
ANALYTICAL CHEMISTRY
Analytical chemistry involves the qualitative and quantitative determination of the chemical components of substances.
PHYSICAL CHEMISTRY
BIOCHEMISTRY
Read Also: Algebra Road Trip Project Answer Key
Relative Atomic Mass Of An Element
The mass number should also not be confused with the standard atomic weight of an element, which is the ratio of the average atomic mass of the different isotopes of that element to the unified atomic mass unit. The atomic weight is an actual mass , while the mass number is a counted number .
This weighted average can be quite different from the near-integer values for individual isotopic masses. For instance, there are two main isotopes of chlorine: chlorine-35 and chlorine-37. In any given sample of chlorine that has not been subjected to mass separation there will be roughly 75% of chlorine atoms which are chlorine-35 and only 25% of chlorine atoms which are chlorine-37. This gives chlorine a relative atomic mass of 35.5 ” rel=”nofollow”>mol).
Moreover, the weighted average mass can be near-integer, but at the same time not corresponding to the mass of any natural isotope. For example, bromine has only two stable isotopes, 79Br and 81Br, naturally present in approximately equal fractions, which leads to the standard atomic mass of bromine close to 80 , even though the isotope 80Br with such mass is unstable.
Charge Numbers In Nuclear And Hadron Physics
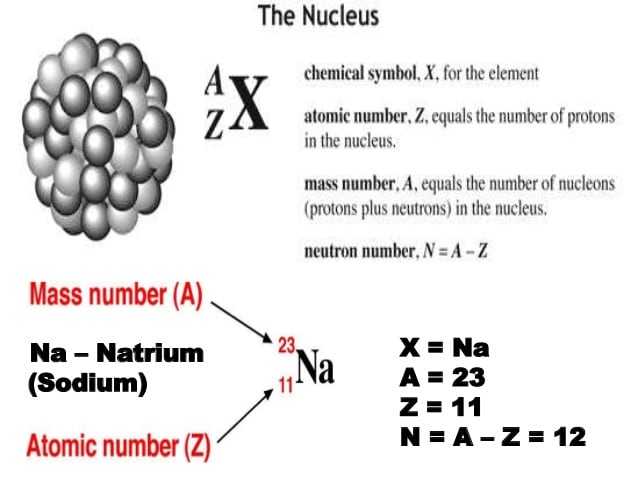
For an atomic nucleus, which can be regarded as an ion having stripped off all electrons, the charge number is identical with the atomic number Z, which corresponds to the number of protons in ordinary atomic nuclei.
Unlike in chemistry, subatomic particles with electric charges of two elementary charges are indicated with a superscript “++” or “ââ”. In chemistry, the same charge numbers are usually indicated as superscript “+2” or “â2”.
Also Check: How To Calculate Frequency Physics
Charge Numbers In Chemistry
Charge number or valence of an ion is the coefficient that, when multiplied by the elementary charge, gives the ion’s charge.
For example, the charge on a chloride ion, C e , where e is the elementary charge. This means that the charge number for the ion is â
z is used as the symbol for the charge number. In that case, the charge of an ion could be written as Q .
The charge number in chemistry normally relates to an electric charge. This is a property of specific subatomic atoms. These elements define the electromagnetic contact between the two elements.
A chemical charge can be found by using the periodic table. An element’s placement on the periodic table indicates whether its chemical charge is negative or positive. Looking at the table, one can see that the positive charges are on the left side of the table and the negative charges are on the right side of the table. Charges that are positive are called cations. Charges that are negative are called anions. Elements in the same group have the same charge. A group in the periodic table is a term used to represent the vertical columns.
- NH
are salts.
Charge numbers also help to determine other aspects of chemistry. One example is that someone can use the charge of an ion to find the oxidation number of a monatomic ion. For example, the oxidation number of Li }} is +1. This helps when trying to solve oxidation questions.
The Discovery Of The Neutron Makes Z The Proton Number
All consideration of nuclear electrons ended with James Chadwick‘s discovery of the neutron in 1932. An atom of gold now was seen as containing 118 neutrons rather than 118 nuclear electrons, and its positive charge now was realized to come entirely from a content of 79;protons. After 1932, therefore, an element’s atomic number Z was also realized to be identical to the proton number of its nuclei.
You May Like: What Is Cardinal Direction In Geography
A Problem In Naming Geometric E
Consider a simple example of 1,2-dichloroethene. The geometric isomerism is given below:
Onecan easily observe which one is cis and which is trans just in one glance. Youmust remember that trans means across and cis meansopposite. This is a simple visual way of observing the two isomersthan why do we need another system?
The problem arises when the compound gets more complicated. For example, are you able to name the isomers given below by using cis and trans?
Thereason is that everything attached to the carbon-carbon double bond isdifferent, the way they look doesnt make obvious that they are being cis ortrans to each other.
Look Up Definitions Of Important Chemistry Terms
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
This alphabetical chemistry dictionary offers definitions and examples of important chemistry and chemical engineering terms. For each term, a brief definition is given. Each link leads to a more comprehensive discussion of the word. Additional definitions are also available.
Also Check: What Kind Of Math Is On The Ged Test
What Are Z Isomers
Z isomers are alkenes having the substituents with higher priority on the same side of the double bond. The letter Z comes from zusammenin German, which means together.
Figure 02: E-Z nomenclature of 2-butene
In the above image, the substituents with high priority are on the same side of the double bond in the Z isomer whereas the E isomer has those substituents in the opposite sides. Furthermore, the CIP rules determine the priority of these substituents. For the above example, the atoms directly bonded to the double bonded carbon are Carbon atoms of methyl groups and hydrogen atoms . Since carbon atom has a high atomic number when compared with hydrogen , the high priority is to the methyl group .
For A Compound Or Mixture
An alternative definition of the effective atomic number is one quite different from that described above. The atomic number of a material exhibits a strong and fundamental relationship with the nature of radiation interactions within that medium. There are numerous mathematical descriptions of different interaction processes that are dependent on the atomic number, Z. When dealing with composite media , one therefore encounters the difficulty of defining Z. An effective atomic number in this context is equivalent to the atomic number but is used for compounds and mixtures of different materials . This is of most interest in terms of radiation interaction with composite materials. For bulk interaction properties, it can be useful to define an effective atomic number for a composite medium and, depending on the context, this may be done in different ways. Such methods include a simple mass-weighted average, a power-law type method with some relationship to radiation interaction properties or methods involving calculation based on interaction cross sections. The latter is the most accurate approach , and the other more simplified approaches are often inaccurate even when used in a relative fashion for comparing materials.
In many textbooks and scientific publications, the following – simplistic and often dubious – sort of method is employed. One such proposed formula for the effective atomic number, Zeff, is as follows :
- Z
Read Also: What Is Activation Energy Biology
The Proton And The Idea Of Nuclear Electrons
In 1915, the reason for nuclear charge being quantized in units of Z, which were now recognized to be the same as the element number, was not understood. An old idea called Prout’s hypothesis had postulated that the elements were all made of residues of the lightest element hydrogen, which in the Bohr-Rutherford model had a single electron and a nuclear charge of one. However, as early as 1907, Rutherford and Thomas Royds had shown that alpha particles, which had a charge of +2, were the nuclei of helium atoms, which had a mass four times that of hydrogen, not two times. If Prout’s hypothesis were true, something had to be neutralizing some of the charge of the hydrogen nuclei present in the nuclei of heavier atoms.
Examples Of Chemistry In A Sentence
chemistrychemistrychemistrychemistry clevelandchemistry Washington Postchemistry Detroit Free Presschemistry CNNchemistrySan Diego Union-Tribunechemistry Chronchemistry BostonGlobe.comchemistryUSA TODAY
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘chemistry.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Read Also: How To Get Answers On Delta Math
The Periodic Table And A Natural Number For Each Element
Loosely speaking, the existence or construction of a periodic table of elements creates an ordering of the elements, and so they can be numbered in order.
Dmitri Mendeleev claimed that he arranged his first periodic tables in order of atomic weight . However, in consideration of the elements’ observed chemical properties, he changed the order slightly and placed tellurium ahead of iodine . This placement is consistent with the modern practice of ordering the elements by proton number, Z, but that number was not known or suspected at the time.
A simple numbering based on periodic table position was never entirely satisfactory, however. Besides the case of iodine and tellurium, later several other pairs of elements were known to have nearly identical or reversed atomic weights, thus requiring their placement in the periodic table to be determined by their chemical properties. However the gradual identification of more and more chemically similar lanthanide elements, whose atomic number was not obvious, led to inconsistency and uncertainty in the periodic numbering of elements at least from lutetium onward .
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie; no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Recommended Reading: How To Do Friction Problems In Physics
Heres My Simple Approach
Naming E And Z Alkenes
The E and Z designation for the configuration of a double bond is also included in the nomenclature of alkenes.
As an example, lets name the alkene for which we have just determined the E/Z configuration above:
First, determine the name according to the IUPAC rules. The parent chain is heptane and there are three substituents:
Additionally, the configuration of the alkene is placed before the systematic name:
;Below are some practice problems for determining the E and Z configuration of alkenes:
Read Also: What Is Shadowing In Psychology
Summary E Vs Z Isomers
E-Z notation or nomenclature is used to name isomers having the same molecular formula and spatial structure, giving each isomer a uniqueness. The difference between E and Z isomers is that the E isomers have the substituents with higher priority in the opposite sides whereas the Z isomers have the substituents with higher priority on the same side.
Reference:
1. E-Z Notation. Wikipedia, Wikimedia Foundation, 10 Apr. 2018. Available here ;2. Libretexts. 19.7: E,Z Notation. Chemistry LibreTexts, Libretexts, 26 June 2017. Available here;3. Libretexts. 3.6 Cahn-Ingold Prelog Rules. Chemistry LibreTexts, Libretexts, 21 July 2016. Available here;
Image Courtesy:
1.EZNotationBy Pete Davis Own work, via Commons Wikimedia;2.EZNotationBy Pete Davis Own work, via Commons Wikimedia ;
Difference Between E And Z Isomers
April 23, 2018 Posted by Madhu
The key difference between E and Z isomers is that E isomers have the substituents with higher priority in the opposite sides whereas the Z isomers have the substituents with higher priority on the same side.
The E-Z nomenclature is a notation system to name different isomers having the same chemical formula, but different spatial arrangements. Furthermore, the E and Z isomers are alkenes. These isomers get their name based on the position of the substituents attached to the double bond of the alkene.
Also Check: Mcdougal Littell Geometry 9.5 Answers
Determining E And Z Configuration
So, how is the E and Z configuration of a double bond determined?
It is determined based on the priorities of the groups on the double bond:
Here is the principle; you need to look at each carbon of the double bond separately. And the goal is to first determine which of the two groups on each carbon has a higher priority.
The priorities are assigned following the same rules for the R and S configuration.
For example, we have seen that this alkene cannot be classified as cis or trans but is it E or Z?
Lets first focus on the left carbon. It has an ethyl group and Cl connected to it. The Cl has a higher priority because of its atomic number:
On the right carbon, we need to compare a hydrogen with a Br atom. Clearly, the Br has a higher priority:
Finally, determine whether the higher priority group on each carbon is on the same or opposite side of the double bond. Since Cl and Br are pointing up and down, they are on opposite sides and the alkene has an E configuration:
You may wonder how the Z configuration of this alkene wouldve looked like. Right below:
How do I remember that it is Z when the groups are on the same and E when they are on opposite sides?
Use this handy trick:Z stands for Zame-they are on the zame side. This might be enough to figure out the E as well, however, you go withE as Epposite sides of the double bond.