What Does An E At The End Of A Number Mean
Uppercase “E” stands for “exponent” in calculator displays. Calculator manufacturers use it to display numbers in scientific notation because the longhand version is difficult to display and would be even more difficult to read. To complicate matters, some calculator manufacturers use lowercase “e” to denote exponents, inviting confusion between scientific notation and Euler’s number, which is a different thing altogether. Don’t be taken in. If either an uppercase or lowercase letter “e” appears on your display, it denotes an exponent. The only place you’ll see Euler’s number is on the keypad.
TL DR
On a calculator display, E stands for exponent of 10, and it’s always followed by another number, which is the value of the exponent. For example, a calculator would show the number 25 trillion as either 2.5E13 or 2.5e13. In other words, E is a short form for scientific notation.
Cis Trans And E Z Geometric Isomers
Your organic chemistry course will cover many different types of isomers.
Isomers have the same molecular formula but something about them is different.Geometric isomers, a type of stereoisomer, differ in their geometry or shape. This happens when substituents are LOCKED in a specific relationship to each other.
I say locked because, unlike conformational isomers in Newman Projections, you cant simply rotate the molecule to change the relationship between substituents.
In this tutorial, well look at alkene geometric isomers including cis trans and E Z.
What Is The Name Of The Formula E = Q + W
What is the name of this formula? Google could not provide me with an answer.
$$\Delta E=q+w$$
- $\begingroup$try $$\Delta Q = \Delta U + \Delta W $$$\endgroup$
This is a statement of the first law of thermodynamics. The first law says that the energy of an isolated system is conserved. An isolated system is one in which neither mass nor energy is exchanged with the surroundings. This equation shows the two means by which a closed system can exchange energy with its surroundings – by heat, or by work.
$$\Delta E=q+w$$
All energy transfers can be described as either heat flow or as work done on or by a system, and therefore the total amount of heat energy that goes in or out , plus the total amount of work energy that goes in or out , must be equal to the total internal energy change of the system .
This equation is particularly important in chemical thermodynamics because we have no way of measuring the total internal energy of a real system directly – all that we can measure is the amount of heat and work that goes in or out of the system, and therefore the change in internal energy.
- 3$\begingroup$To be more precise, the expression of the first law we are talking about in this thread works for closed systems, not for non-isolated , because for an open system energy can also be transferred by the third way: by adding/removing matter.$\endgroup$ WildcatAug 10, 2014 at 5:58
There are several forms of expression of the first law of thermodynamics:
As well as:
Also Check: Linear Algebra Definition Of Span
Key Concepts And Summary
Chemists use nomenclature rules to clearly name compounds. Ionic and molecular compounds are named using somewhat-different methods. Binary ionic compounds typically consist of a metal and a nonmetal. The name of the metal is written first, followed by the name of the nonmetal with its ending changed to ide. For example, K2O is called potassium oxide. If the metal can form ions with different charges, a Roman numeral in parentheses follows the name of the metal to specify its charge. Thus, FeCl2 is iron chloride and FeCl3 is iron chloride. Some compounds contain polyatomic ions the names of common polyatomic ions should be memorized. Molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a molecule of the compound. Examples include SF6, sulfur hexafluoride, and N2O4, dinitrogen tetroxide. Acids are an important class of compounds containing hydrogen and having special nomenclature rules. Binary acids are named using the prefix hydro-, changing the ide suffix to ic, and adding acid HCl is hydrochloric acid. Oxyacids are named by changing the ending of the anion to ic, and adding acid H2CO3 is carbonic acid.
Chemically Pure And Isotopically Pure
Chemists and nuclear scientists have different definitions of a pure element. In chemistry, a pure element means a substance whose atoms all have the same atomic number, or number of protons. Nuclear scientists, however, define a pure element as one that consists of only one stable isotope.
For example, a copper wire is 99.99% chemically pure if 99.99% of its atoms are copper, with 29 protons each. However it is not isotopically pure since ordinary copper consists of two stable isotopes, 69% 63Cu and 31% 65Cu, with different numbers of neutrons. However, a pure gold ingot would be both chemically and isotopically pure, since ordinary gold consists only of one isotope, 197Au.
Also Check: Kuta Software Similar Triangles Answer Key
Euler’s Number In Nature
Exponents with e as a base are known as natural exponents, and here’s the reason. If you plot a graph of
you’ll get a curve that increases exponentially, just as you would if you plotted the curve with base 10 or any other number. However, the curve y = ex has two special properties. For any value of x, the value of y equals the value of the slope of the graph at that point, and it also equals the area under the curve up to that point. This makes e an especially important number in calculus and in all the areas of science that use calculus.
The logarithmic spiral, which is represented by the equation
is found throughout nature, in seashells, fossils and and flowers. Moreover, e turns up in numerous scientific contexts, including the studies of electric circuits, the laws of heating and cooling, and spring damping. Even though it was discovered 350 years ago, scientists continue to find new examples of Euler’s number in nature.
Related Articles
What If Theres More Than One Substituent On The Sp2 Carbon
Till now, weve looked at molecules with just one substituent on either side of the sp2 pi bound carbon.
What happens if we have a pi bond with 2 different atoms or groups on the sp2 carbon?
Take a look at 3-methyl-2-pentene:
Here in line structure:
You can draw this molecule in 2 different ways. But will you compare the red methyl or red ethyl to the green methyl when choosing cis or trans?
While some professors WILL teach you to compare the larger groups, the answer is that you CANNOT compare simply choose one for cis and trans.
Recommended Reading: How To Find The Indicated Length
Anatomy Of An Equation
Hydrogen gas and chlorine gas will react vigorously to produce hydrogen chloride gas. The equation above illustrates this reaction. The reactants, hydrogen and chlorine, are written on the left and the products on the right. The large number 2 in front of HCl indicates that two molecules of HCl are produced for each 1 molecule of hydrogen and chlorine gas consumed. The 2 in subscript below H indicates that there are two hydrogen atoms in each molecule of hydrogen gas. Finally, the symbols subscript to each species indicates that they are gases.
Species in a chemical reaction is a general term used to mean atoms, molecules or ions. A species can contain more than one chemical element . Each species in a chemical equation is written:
E is the chemical symbol for the element, x is the number of atoms of that element in the species, y is the charge and is the physical state.
The symbols in parentheses indicate the physical state of each reactant or product. For ACS Style the state is typeset at the baseline without size change.
- means solid
- means aqueous solution
For example, ethyl alcohol would be written C ) }_}_}_} because each molecule contains 2 carbon, 6 hydrogen and 1 oxygen atom. A magnesium ion would be written Mg + }^} because it has a double positive charge. Finally, an ammonium ion would be written NH + }_^}
Compounds Composed Of Two Elements
When two nonmetallic elements form a molecular compound, several combination ratios are often possible. For example, carbon and oxygen can form the compounds CO and CO2. Since these are different substances with different properties, they cannot both have the same name . To deal with this situation, we use a naming method that is somewhat similar to that used for ionic compounds, but with added prefixes to specify the numbers of atoms of each element. The name of the more metallic element is first, followed by the name of the more nonmetallic element with its ending changed to the suffix ide. The numbers of atoms of each element are designated by the Greek prefixes shown in Table 11.
| Number |
|---|
| Table 14. Names of Common Oxyacids |
Also Check: Molecular Shape For Ccl4
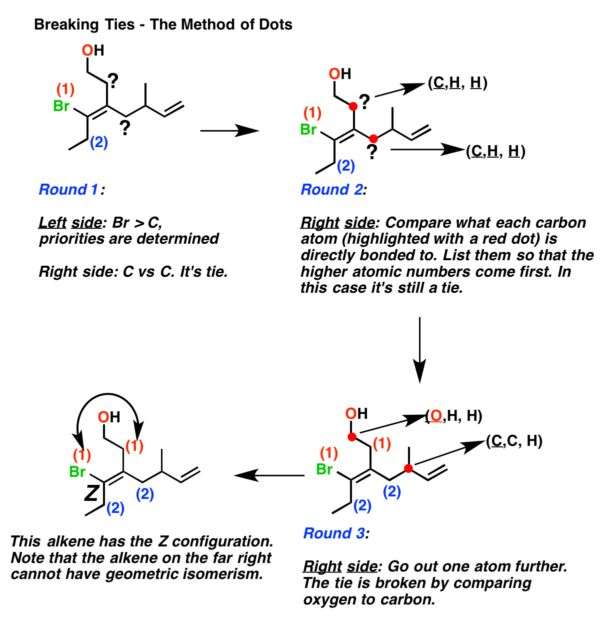
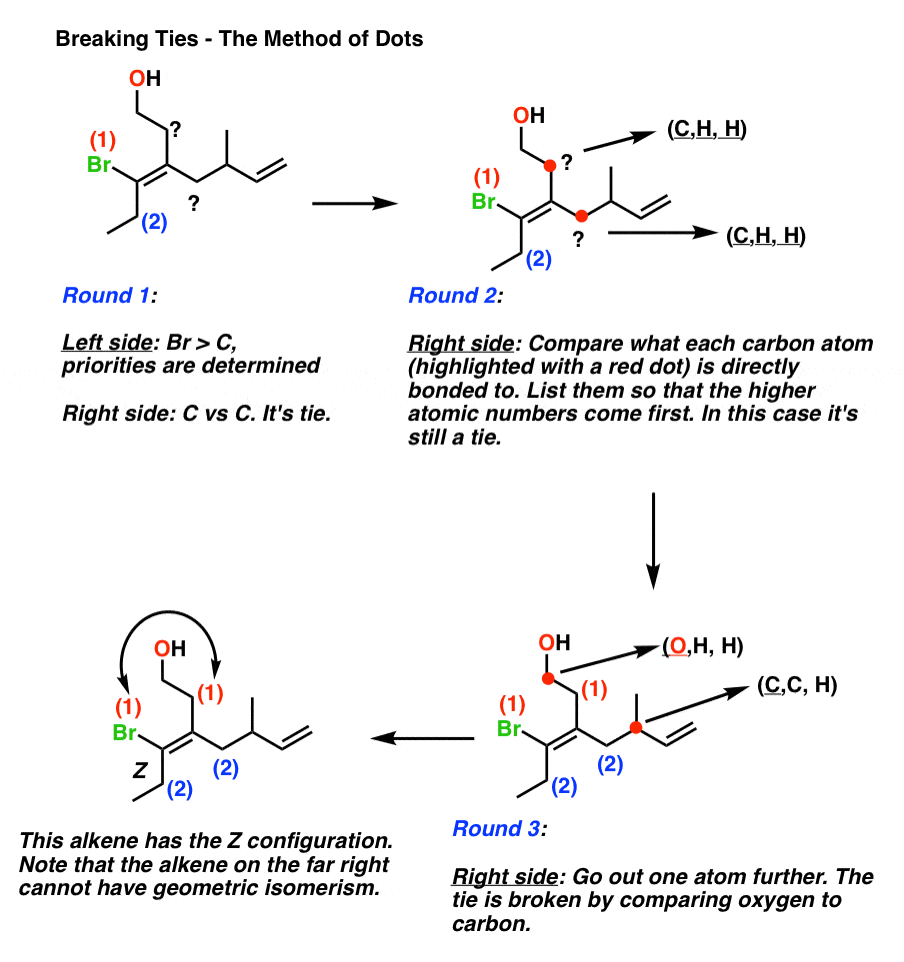
Introducing The E Z Notation
When a pi bond has more than one substituent on each side, or contains non-carbon substituents, well need a more advanced system for identifying geometric isomerism.
The E Z system requires ranking the groups on either side of the pi bond. We must determine if the higher priority groups are next to each other, Z , or away from each other, E .
Th Century And Recent Developments
In 1902, The Electrochemical Society was founded.
In 1909, Robert Andrews Millikan began a series of experiments to determine the electric charge carried by a single electron.In 1911, Harvey Fletcher, working with Millikan, was successful in measuring the charge on the electron, by replacing the water droplets used by Millikan, which quickly evaporated, with oil droplets. Within one day Fletcher measured the charge of an electron within several decimal places
In 1923, Johannes Nicolaus Brønsted and published essentially the same theory about how acids and bases behave, using an electrochemical basis.
In 1937, Arne Tiselius developed the first sophisticated electrophoretic apparatus. Some years later, he was awarded the 1948 Nobel Prize for his work in protein electrophoresis.
A year later, in 1949, the International Society of Electrochemistry was founded.
By the 1960sâ1970s quantum electrochemistry was developed by Revaz Dogonadze and his students.
Recommended Reading: Geometry Of Ccl4
What Is E=hv In Chemistry
E=hv is used to calculate the energy of electromagnetic radiation, and is known as the Planck-Einstein relation.E is the energy of electromagnetic radiation, h is the Planck constant and v is the frequency of electromagnetic radiation.
In 1900, Planck introduced the hypothesis that energy is emitted in quanta, instead of a continuous emission. He proposed the equation E=hv, stating that a quantum of energy is related to the frequency. He claimed that the processes of absorption and emission of radiation had nothing to do with the physical reality of the radiation itself. In 1905, Einstein reinterpreted Plancks hypothesis and used it to explain the photoelectric effect.
The Relationship Between Cell Potential & Gibbs Energy
Electrochemical cells convert chemical energy to electrical energy and vice versa. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends on both the cell potential and the total number of electrons that are transferred from the reductant to the oxidant during the course of a reaction. The resulting electric current is measured in coulombs , an SI unit that measures the number of electrons passing a given point in 1 s. A coulomb relates energy to electrical potential . Electric current is measured in amperes 1 A is defined as the flow of 1 C/s past a given point :
In chemical reactions, however, we need to relate the coulomb to the charge on a mole of electrons. Multiplying the charge on the electron by Avogadros number gives us the charge on 1 mol of electrons, which is called the faraday , named after the English physicist and chemist Michael Faraday :
\ & =9.64833212\times10^4\textrm^- \\ & \simeq 96,485\, J/\end \label\]
The total charge transferred from the reductant to the oxidant is therefore \, where \ is the number of moles of electrons.
Michael Faraday
The maximum amount of work that can be produced by an electrochemical cell ) is equal to the product of the cell potential ) and the total charge transferred during the reaction ):
Work is expressed as a negative number because work is being done by a system on its surroundings.
Example \
Given: redox reaction
Read Also: The Founder Of Behaviorism Was:
When Do We Use Cis
We use the terms cis- and trans to denote the relative configuration of two groups to each other in situations where there is restricted rotation.
In nomenclature, cis is used to distinguish the isomer where two identical groups are pointing in the same direction from the plane of the ring, and trans to distinguish the isomer where they point in opposite directions.
A common name for these so-called cis-trans isomers is geometric isomers.
In order for cis- trans- isomerism to exist in rings, we need two conditions:
- two carbons each bearing non-identical substituents above and below the ring
- the two carbons have at least one of those substituents in common
In 1,2-dichlorocyclopentane we saw that C-1 and C-2 each had non-identical substituents above and below the ring, and they each had at least one substituent in common .
Heres another example: cis- and trans 1-ethyl-2-methylcyclobutane. Note that they each have two carbons which each bear non-identical substituents above and below the ring . They also have at least one substituent in common . So we can refer to cis-1-ethyl-2-methylcyclohexane as the isomer where the two hydrogens are pointing in the same direction, and trans where they point in opposite directions.
If youve covered chirality, you might also note an interesting fact: there are two ways to draw each of the cis- and trans isomers, and they cant be superimposed on each other. These are enantiomers, by the way.
Conclusion: E And Z Notation For Alkenes
cis-trans- is OK for describing simple alkene stereoisomers, but only works in certain cases. Furthermore, it only gives relative configurations. The E/Z system is comprehensive and describes the absolute configuration of the molecule.
See below for an example of an E alkene which is cis and a Z alkene which is trans.
Just a reminder: this post was co-authored by Matt Pierce of Organic Chemistry Solutions. Ask Matt about scheduling an online tutoring session here.
You May Like: How To Find The Half Life
Gibbs Free Energy And Cell Potential
GTRQ
A typical galvanic electrochemical cell: Under standard conditions, the output of this pair of half-cells is well known. When a change in the concentration or activity of reactants occurs, or the temperature or pressure changes, the output voltage changes. It is calculated via the Nernst equation.
The E And Z Notation For Alkenes
Thankfully, we can apply the ranking system developed by Cahn, Ingold, and Prelog for chiral centers / nomenclature) for this purpose.
The protocol is as follows:
- Each carbon in the pi bond is attached to two substituents. For eachcarbon, these two substituents are ranked according to the atomic numbers of the atom directly attached to the carbon.
- If both substituents ranked 1 are on the same side of the pi bond, the bond is given the descriptor Z .
- If both substituents ranked 1 are on the opposite side of the pi bond, the bond is given the descriptor E .
So Z resembles cis and E resembles trans . (Note: they are not necessarily the same and do not always correlate: see footnote for an example of a cis alkene which is E . The E/Z system is comprehensive for all alkenes capable of geometric isomerism, including the cis/trans alkene examples above. We often use cis/trans for convenience, but E/Z is the official, IUPAC approved way to name alkene stereoisomers].
One easy way to remember Z is to say Zee Zame Zide in a German accent. My way of doing it was pretending that the Z stands for zis. Whatever works for you.
Heres a practical example:
As with chiral centers, ranking according to atomic number can result in ties if we restrict ourselves merely to the atoms directly attached to the pi bonds.
Also Check: Geography Movement Definition
Corrosion Of Common Metals
Coinage metals, such as copper and silver, slowly corrode through use.A patina of green-blue copper carbonate forms on the surface of copper with exposure to the water and carbon dioxide in the air. Silver coins or cutlery that are exposed to high sulfur foods such as eggs or the low levels of sulfur species in the air develop a layer of black silver sulfide.
Gold and platinum are extremely difficult to oxidize under normal circumstances, and require exposure to a powerful chemical oxidizing agent such as aqua regia.
Some common metals oxidize extremely rapidly in air. Titanium and aluminium oxidize instantaneously in contact with the oxygen in the air. These metals form an extremely thin layer of oxidized metal on the surface, which bonds with the underlying metal. This thin oxide layer protects the underlying bulk of the metal from the air preventing the entire metal from oxidizing. These metals are used in applications where corrosion resistance is important. Iron, in contrast, has an oxide that forms in air and water, called rust, that does not bond with the iron and therefore does not stop the further oxidation of the iron. Thus iron left exposed to air and water will continue to rust until all of the iron is oxidized.
Ecell: Standard Cell Potential
E°cell is the electromotive force between two half-cells. The greater the E°cell of a reaction the greater the driving force of electrons through the system, the more likely the reaction will proceed . E°cell is measured in volts . The overall voltage of the cell = the half-cell potential of the reduction reaction + the half-cell potential of the oxidation reaction. To simplify,
The potential of an oxidation reduction is the negative of the potential for a reduction potential . Most tables only record the standard reduction half-reactions as standard reduction potentials. To find the standard oxidation potential, simply reverse the sign of the standard reduction potential.
Note
The more positive reduction potential of reduction reactions are more spontaneous. When viewing a cell reduction potential table, the higher the cell is on the table, the higher potential it has as an oxidizing agent.
Also Check: How To Calculate Half Life Chemistry