What Is A Pseudohalogen/pseudohalide
What do you mean by a pseudohalogen/pseudohalide and how do you tell whether a molecule/ion is a pseudohalogen/pseudohalide ?
Pseudohalides are “fake halides.” Their chemistry bears some resemblance to true halides .
Examples of pseudohalides include $\ce$ and $\ce$. These are generally weak Lewis bases that bear a formal negative 1 charge.
In my opinion, it is rather subjective as to what can be labeled as a pseudohalide. In general, it is in the eye of the beholder, and for a certain set of chemical circumstances.
In short, there is no “dividing line” for being a pseudohalide. If we assume that halides undergo certain types of chemistry, and the species X in question also generally does those same types of chemistry with a similar mechanism, then you may be able to label X a “pseudohalide.”
- $\begingroup$But F-,Cl- tend to make low-spin complexes and CN- ion tends to make a high-spin complex. Now considering this difference isn’t ‘pseudohalogen’ a misnomer ?$\endgroup$May 29 ’13 at 15:41
- 1$\begingroup$Yes, it is probably a misnomer in the context of coordination chemistry, that you cited here. But, it may have certain aspects of acid/base chemistry that look like halogens. BTW, I think it’s kind of a silly concept: pseudohalides. It’s just asking for a fight as to whether group X is enough like any/all/some halides in all reactivities/spectroscopies to be called “pseudohalide.” Truth is, X is a halide or it is not.$\endgroup$
Rearrangement Of Acyl Azides: The Curtius Rearrangement
The most common use for acyl azides is that upon heating, they rearrange to give isocyanates in a reaction known as the Curtius rearrangement.
- If the Curtius is performed in the presence of an alcohol such as methanol, a carbamate is formed.
- If water is added instead, an unstable carbamic acid is briefly formed, which then loses carbon dioxide to give a primary amine.
Why might this be useful? Heres one way.
There are a lot of methods for making aromatic carboxylic acids, but not so many great ways of forming C-N bonds on aromatic rings. Say you have a benzoic acid but need to form a aromatic amine. The Curtius protocol represents a nice way of forming a new C-N bond on the ring without having to resort to nitration + reduction.
Nucleophilic Acyl Substitution With Azide Salts Makes Acyl Azides
The SN2 reaction isnt the only type of substitution reaction weve explored. Theres also nucleophilic acyl substitution. A carbonyl attached to a good leaving group will undergo substitution when an appropriate nucleophile is added.
In the first step of the mechanism, the nucleophile attacks the carbonyl carbon, forming C-Nu and breaking C-O . This gives rise to a tetrahedral intermediate. In the second step, the C-O pi bond is re-formed, and the carbon- bond is broken, resulting in the nucleophilic acyl substitution product.
Here is an example of a nucleophilic acyl substitution reaction between an acid chloride and the N3 ion to give an acyl azide:
Read Also: What Is Abundance In Chemistry
Total Number Of Electrons Of The Valance Shells Of N3
Nitrogen is a group VA element in the periodic table and contains five electrons in its last shell.
To find out total valence electrons given by a particular element, you should multiply number of electrons of the valance shell by the number of atoms of that element in respective molecule.
- valence electrons given by nitrogen atoms = 5*3 = 15
Because there is a -1 charge in N3- ion, an extra electron is received to total valence electrons.
- electrons received due to -1 charge = 1
- Total valence electrons = 15 + = 16
Are Three A Triple Bond In N3
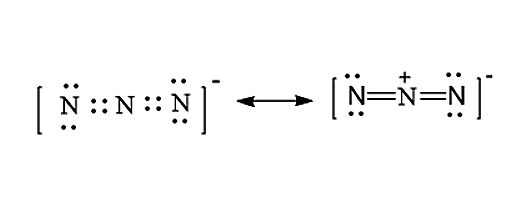
No, If we put a triple bond between two nitrogen atoms, we have to face a problem of one nitrogen atom having -2 charge and center nitrogen atom has +1 charge. That kind of charge distribution is not stable for a molecule or ion. Thereofore, having a triple bond between two nitrogen atoms is not possible in N3- lewis structure.
Also Check: What Is Percentage Error In Chemistry
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Don’t Miss: Geometry Dash Practice Song Hack
Organic Azides As Masked Amines
Note the resonance form above with a nitrogen-nitrogen triple bond. If treated with a reducing agent, such as LiAlH4 or even catalytic hydrogenation organic azides can be reduced to primary amines, liberating N2 in the process.
This makes for a very useful route to primary amines from alkyl halides!
Weve previously explored the Gabriel synthesis as a route to primary amines , but this route is superior because the azide can be reduced to the amine under gentle conditions . None of that high-temperature cleavage of the phthalimide with hydrazine to worry about.
We also saw that making primary amines through direct treatment of alkyl halides with NH3 often doesnt result in the desired product, because of amines have a Cookie-Monster-like tendency to react multiple times with alkyl halides: since the amine products tend to be more nucleophilic than the reactants, its hard to get amines to stop at munching just one alkyl halide.
What Is The Correct Name For The N3
| azide Azide ion Hydrazoate Azide anion 14343-69-2 More |
| Molecular Weight |
What is the correct name for the nitrogen ion?,
| Element name | |
| Phosphide | P3 |
Furthermore, Which statement is correct for N3 ion?, The statement true for N-3 is. The azide ion has 16 valence electrons and is isoelectronic with CO2. The N-3 ion is linear like CO2 molecule. The formal oxidation state of nitrogen in this anion is -1, because the net charge on the ion is -1 as shown in the following resonating structures.
Finally, How does the N-3 ion form?, Valence Shell Electrons
Therefore, the N3 ion is the most common ion formed from a single nitrogen atom. So, although it is much rarer than a nitrogen atom gaining three electrons and becoming N3, it can also lose three or five electrons to form one of two cations: N3+ or N5+.
You May Like: Geometry Basics Segment Addition Postulate Worksheet Answer Key
Mechanism Of The Copper
As one of the best click reactions to date, the copper-catalyzed azide-alkyne cycloaddition features an enormous rate acceleration of 107to 108 compared to the uncatalyzed 1,3-dipolar cycloaddition. It succeeds over a broad temperature range, is insensitive to aqueous conditions and a pH range over 4 to 12, and tolerates a broad range of functional groups. Pure products can be isolated by simple filtration or extraction without the need for chromatography or recrystallization.
F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless, V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210-216.
The active Cu catalyst can be generated from Cu salts or Cu salts using sodium ascorbate as the reducing agent. Addition of a slight excess of sodium ascorbate prevents the formation of oxidative homocoupling products. Disproportionation of a Cu salt in presence of a Cu wire can also be used to form active Cu.
DFT calculations have shown that coordination of Cu to the alkyne is slightly endothermic in MeCN, but exothermic in water, which is in agreement with an observed rate acceleration in water. However, coordination of Cu to the acetylene does not accelerate a 1,3-dipolar cycloaddition. Such a process has been calculated to be even less favorable than the uncatalyzed 1,3-dipolar cycloaddition.
B. T. Worell, J. A. Malik, V. V. Fokin, Science, 2013, 340, 457-460. DOI:10.1126/science.1229506
Drawing The Lewis Structure For N3
Video: Drawing the Lewis Structure for N3-
In the Lewis Structure for N3- you’ll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule.
For the N3- Lewis structure, calculate the total number of valence electrons for the N3- molecule. After determining how many valence electrons there are in N3- , place them around the central atom to complete the octets. Be sure to use the number of available valence electrons you found earlier.
There are 16 valence electrons for the Lewis structure for N3-.
You should take formal charges into account with the Lewis structure for N3- to find the best structure for the molecule. Also note that you should put the N3- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
It is helpful if you:
- Try to draw the N3- Lewis structure before watching the video.
- Watch the video and see if you missed any steps or information.
- Try structures similar to N3- for more practice.
Also Check: Age Word Problem With Solution
The Azide Ion Is A Great Nucleophile In Sn2 Reactions
The azide ion is the conjugate base of hydrazoic acid, HN3. Despite being only weakly basic N3 is an extremely good nucleophile according to one measure, more nucleophilic than any amine . Its not hard to rationalize why this might be when you stop to think about it. With four nucleophilic lone pairs confined to a very small volume, the likelihood of a collision with an electrophile that results in a reaction is much higher than it would be for an amine with bulky alkyl groups, to take just one example.
You can think of N3 as a lean, mean, nitrogen-tipped missile that delivers its payload onto carbon quickly, efficiently, and without significant side reactions.
In SN2 reactions, primary and secondary alkyl halides and sulfonates are readily displaced by N3 , resulting in alkyl azides:
The usual procedure is to use an azide salt such as NaN3 or KN3 with the appropriate alkyl halide in a polar aprotic solvent such as acetonitrile or dimethylsulfoxide .
The organic azide products are reasonably stable, even if they look a little weird. Some have even found use as pharmaceuticals or as useful probes for studying chemical biology. .
For our purposes the most useful property of organic azides is that they serve as masked amines.
Mechanism Of The Huisgen Azide
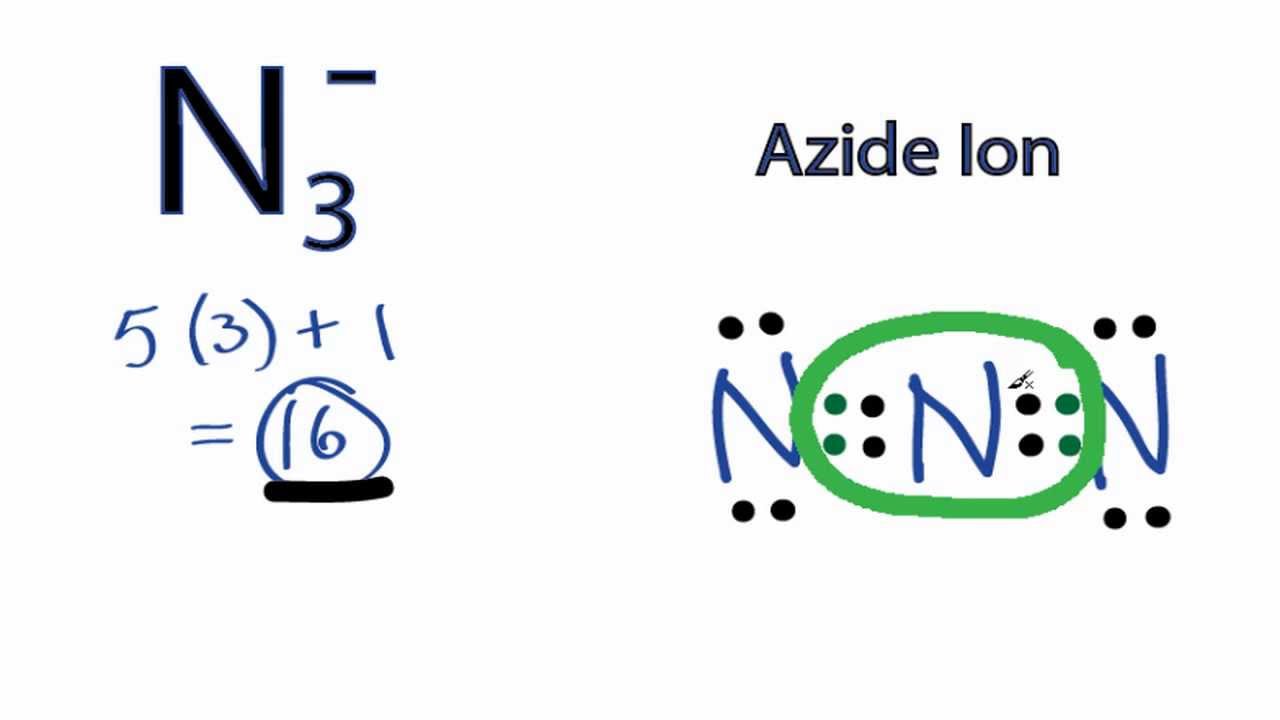
For the mechanism, please refer to the text on1,3-dipolar cycloaddition. This reaction is highly exothermic, but the high activation barrier is responsible for a very low reaction rate, even at elevated temperature. Another drawback is the formation of regioisomers, as the two possible HOMO-LUMO interactions of the substrates are closely related in terms of energy. The thermal reaction therefore often gives approximately 1:1 mixtures of both the 1,4-substituted and the 1,5-substituted regioisomers.
V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. Int. Ed., 2002,41, 2596-2599.
Also Check: Unit 1 Test Study Guide Geometry Basics
Polyazide Chemistry: The First Binary Group 6 Azides Mo6 W6 And And The And Ions
This work was funded by the Air Force Office of Scientific Research and the National Science Foundation. We thank Prof. Dr. G. A. Olah and Dr. M. Berman, for their steady support, and Dr. R. Wagner for his help and stimulating discussions. Dedicated to Professor Kurt Dehnicke for his lifelong contributions to azide chemistry.
Exploding On The Launchpad
If N3 is a nitrogen-tipped missile, as we commented above, its worth recalling what happens when a missile isnt handled with care: its essentially a bomb.
NaN3 and KN3 are white powders that can be stored indefinitely at room temperature and scooped out at the bench without any trouble.
But when heated or subjected to shock all bets are off. The same goes for exposure of these azide salts to acid, which forms potentially explosive HN3.
And NaN3 and KN3 are the nice azides! If mixed with metal salts of lead, mercury, cadmium, zinc, or silver, even more explosive azide compounds can result. These metal azides are contact explosives, some of which are so sensitive that they will detonate if someone farts twenty feet down the hall. Some crazy chemists even make these things on purpose, but we will not be travelling anywhere near the blast radius of that topic on this blog anytime, ever.
NaN3 and KN3 should be used at reasonably dilute concentrations, behind a blast shield on preparative scale, and incidentally never used with CH2Cl2 solvent, which can result in highly explosive diazidomethane.
Yay for azide salts!
Bottom line: SN2 reactions between alkyl halides or sulfonates with azides are probably the single best way to synthesize primary amines from alkyl halides. It certainly beats the Gabriel synthesis.
Read Also: Why Are There Different Branches Of Chemistry
S Of Drawing The Lewis Structure Of N3
There are few steps to draw a lewis structure of a molecule or ion. Because N3- ion is an ion, all basic steps are used to draw it. So, you can learn good basic things of drawing lewis structures from this example.
What Is The Correct Name For N3
correct name
In this regard, what does n3 mean in chemistry?
Hydrazoate. Azide anion. Nitrogen ion
Similarly, what is the correct name for n3 ion? azide
Likewise, people ask, what is the name of Na+?
Nomenclature of simple ionsNaming the element and adding the word ion forms the cation name. So, Na+ is sodium ion.
Is n3 a cation or anion?
Iron , Sodium , Lead are few examples of cations, while Fluoride , Bromide , Iodide , Nitride and Hydride are examples of anions.
Also Check: Find The Length Indicated Geometry Worksheet Answers
Mechanism Of The Ruthenium
A search for catalysts revealed that pentamethylcyclopentadienyl ruthenium chloride complexes are able to catalyze the cycloaddition of azides to terminal alkynes regioselectively leading to 1,5-disubstituted 1,2,3-triazoles. In addition, RuAAC can also be used with internal alkynes, providing fully substituted 1,2,3-triazoles, which contrasts with CuAAC.
B. C. Boren, S. Narayan, L. K. Rasmussen, L. Zhang, H. Zhao, Z. Lin, G. Jia, V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 8923-8930.
The ruthenium-catalyzed azide-alkyne cycloaddition appears to proceed via oxidative coupling of the azide and the alkyne to give a six-membered ruthenacycle, in which the first new carbon-nitrogen bond is formed between the more electronegative carbon of the alkyne and the terminal, electrophilic nitrogen of the azide. This step is followed by reductive elimination, which forms the triazole product. DFT calculations support this mechanistic proposal and indicate that the reductive elimination step is rate-determining..
B. C. Boren, S. Narayan, L. K. Rasmussen, L. Zhang, H. Zhao, Z. Lin, G. Jia, V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 8923-8930.
Recent Literature
A Novel Approach to 1-Monosubstituted 1,2,3-Triazoles by a Click Cycloaddition/Decarboxylation ProcessM. Xu, C. Kuang, Z. Wang, Q. Yang, Y. Jiang, Synthesis, 2011, 223-228.
The Use of Calcium Carbide in the Synthesis of 1-Monosubstituted Aryl 1,2,3-Triazole via Click ChemistryY. Jiang, C. Kuang, Q. Yang, Synlett, 2009, 3163-3166.