Solubility Of Ionic Compounds In Water
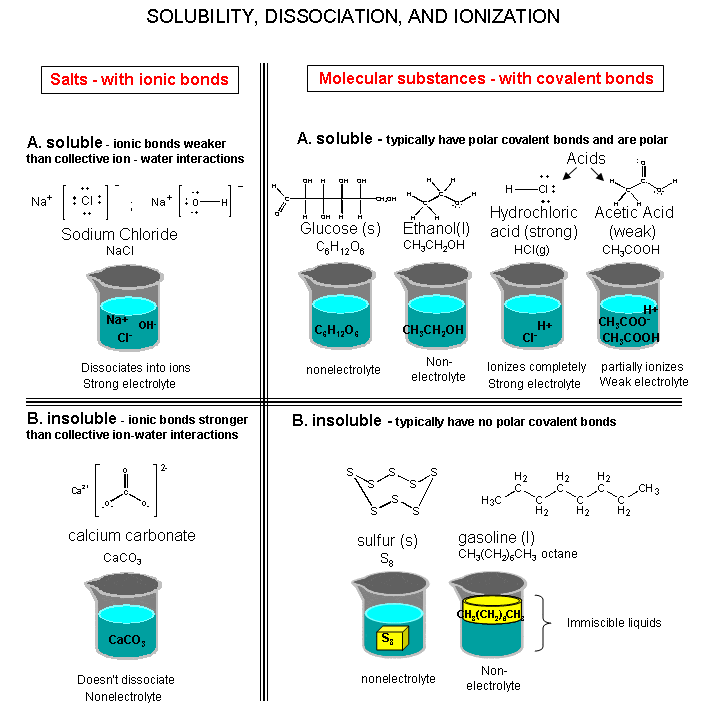
Some ionic compounds dissolve in water, which arises because of the attraction between positive and negative charges . For example, the salt’s positive ions attract the partially negative oxygen atom in H2O. Likewise, the salt’s negative ions attract the partially positive hydrogens in H2O. Note: the oxygen atom is partially negative because it is more electronegative than hydrogen, and vice versa .
- AgCl Ag+ + Cl
However, there is a limit to how much salt can be dissolved in a given volume of water. This concentration is the solubility and related to the solubility product, Ksp. This equilibrium constant depends on the type of salt , temperature, and the common ion effect.
One can calculate the amount of AgCl that will dissolve in 1 liter of pure water as follows:
- Ksp = × / M2
- Ksp = 1.8 × 1010
= , in the absence of other silver or chloride salts, so
- 2 = 1.8 × 1010 M2
- = 1.34 × 105 mol/L
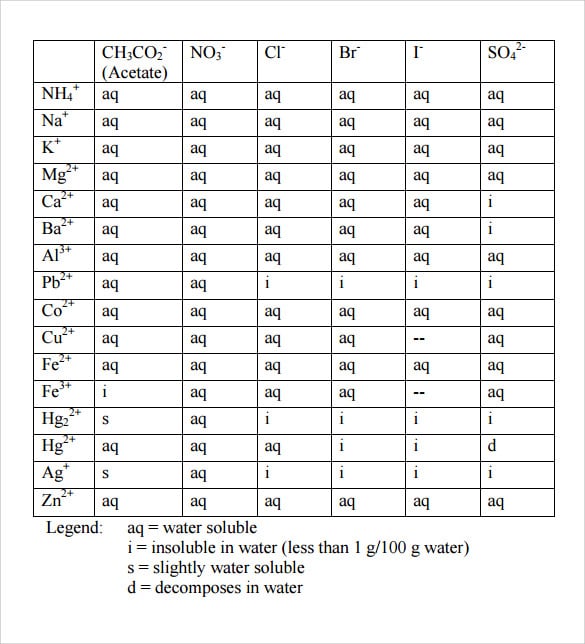
The result: 1 liter of water can dissolve 1.34 × 105moles of AgCl at room temperature. Compared with other salts, AgCl is poorly soluble in water. For instance, table salt has a much higher Ksp = 36 and is, therefore, more soluble. The following table gives an overview of solubility rules for various ionic compounds.
| Soluble |
|---|
| Sulfides |
What Does Insoluble Mean
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Insoluble means incapable of dissolving in a solvent. It is rare for absolutely no solute to dissolve at all. However, many substances are poorly soluble. For example, very little silver chloride dissolves in water, so it is said to be insoluble in water. Note a compound may be insoluble in one solvent yet fully miscible in another. Also, several factors affect solubility. One of the most important is temperature. Increasing temperature frequently improves the solubility of a solute.
Examples Of Soluble In A Sentence
solublesolublesoluble Better Homes & Gardenssoluble Good Housekeepingsoluble Good Housekeepingsoluble SELFsoluble The Salt Lake Tribunesoluble oregonlivesoluble Better Homes & Gardenssoluble CNN
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘soluble.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Also Check: What Is Defense Mechanism In Psychology
What Does Soluble And Insoluble Mean In Chemistry Quora
- Highest rating: 4
- Summary: Solubility is the ability of a solute to dissolve completely in a solvent. When a solute is soluble, it means that it was able
See Details
- Highest rating: 4
- Summary: capable of being solved or explained: a soluble problem. noun. something soluble. QUIZ. THIS QUIZ ON BLUE OPPOSITES WILL SURELY BLUE YOU AWAY. What do you
See Details
- Highest rating: 3
- Summary: Similar term: solubility, solvation, solution. Definition: A substance is soluble if it dissolves in certain fluids. The fluid (present in
See Details
Difference Between Soluble And Insoluble
Soluble vs Insoluble
Solubility and insolubility of material in a solvent is very important. It is even the fundamental phenomenon for the generation of life on earth and the continuation of it. There should be various chemical and physical interactions for a substance to be soluble and insoluble. Here, we will consider these two terms in a broader perspective.
Soluble
Insoluble
Insoluble means that cannot be dissolved. It is the opposite of soluble. As mentioned above, substances dissolve with each other if they like each other. When they dont like each other they are insoluble. In other words, if two substances cannot interact with each other, they wont be soluble. For example, polar substances and non-polar substances do not like each other therefore, there arent any interactions between them. So, non-polar solute will not be soluble in a polar solvent. For example, piece of rubber doesnt soluble in water. Else sugar is not soluble in oil. Insoluble material can be separated easily by filtration method. As there are substances which are completely insoluble, there can be some which are partly soluble. If the solute and the solvent can make interactions for some degree, they are partly soluble.
You May Like: How Is Chemistry Used In Everyday Life
Overview Of Insoluble Solid
A solid is classified as insoluble in a specific liquid if the molecules or ions are not individually detached from the solid and enter into the solution and form bonds with the solvent particles in which we want to form solution. A solid substance might be easily soluble in one solvent but may be totally insoluble in other solvent. Some examples of insoluble substances are wood, sand, metal glass, plastic, and cloth. The mentioned substances are impossible to dissolve in the water at the room temperature.
What Is Insoluble And Soluble Meaning
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
What are soluble substances short answer?
Soluble means capable of being dissolved especially in water. Solubility is the property of a solid, liquid or gaseous substance called solute to dissolve in a solvent forming a solution of solute in the solvent.
What are insoluble substances Class 6?
Insoluble substance is a substance that does not dissolve in a solvent to give a reasonable concentration.
What are insoluble things?
Insoluble generally means that a substance does not dissolve in water. Some examples include: sand, fats, wood, metals, and plastic. When we put them in water and try to mix them, they will not dissolve.
What is insoluble solution?
If a material is insoluble, it does not undergo a change of state. A good example is sand. When put into water, its solid particles mix with liquid particles and they become a solution. Insoluble is not to be confused with soluble materials, which can dissolve in water, such as sugar.
What is the mean by insoluble?
incapable of being dissolvedDefinition of insoluble : not soluble: such as. a : incapable of being dissolved in a liquid and especially water also : soluble only with difficulty or to a slight degree. b : having or admitting of no solution or explanation an insoluble problem.
Recommended Reading: What Does Discriminant Mean In Math
What Does Soluble Mean In Science
Everyday Examples When scientists claim that a substance is soluble, they mean that it can be dissolved, most commonly in water. For example, sodium chloride is soluble in water. Solvents and Solutes In order for a material to be dissolved, there must be a solvent to dissolve it. The solvent in a solution is greater in quantity than the solute. When it is added to the solvent, the solute will have its molecular bonds broken before combining with the solvent. Concentration A solvent can only dissolve so much solute. A solution is unsaturated if you can keep dissolving more solute, whereas it is saturated when the solvent can dissolve no more solute. A supersaturated solution can exist when the solution is heated up to dissolve more solute, increasing the solubility of the solute, then lowering the temperature of the solution. Everyday Examples Solutions are part of everyday life. Coffee is a solution of coffee grounds and water. Automotive antifreeze is mixed with water. Even mixed drinks from the bar are examples of solutions.
Insoluble Let a bottle of salad dressing stand on the table for a few hours and you will notice that it has separated into layers. Thats because oil is insoluble or is not capable of being dissolved.
Video advice: Solubility Rules
Learn the basics about solubility rules for insoluble salts, as part of the overall acids, bases and alkali topic.
Solubility Of Organic Compounds
The principle outlined above under polarity, that like dissolves like, is the usual guide to solubility with organic systems. For example, petroleum jelly will dissolve in gasoline because both petroleum jelly and gasoline are non-polar hydrocarbons. It will not, on the other hand, dissolve in ethyl alcohol or water, since the polarity of these solvents is too high. Sugar will not dissolve in gasoline, since sugar is too polar in comparison with gasoline. A mixture of gasoline and sugar can therefore be separated by filtration or extraction with water.
Also Check: Geometry Creation And Import With Comsol Multiphysics
Solubility Science: How Much Is Too Much
A saturating science project from Science Buddies
Solubility
IntroductionHave you ever added a spoon of sugar to your tea and wondered why it disappeared? Where did it go? The sugar did not actually disappearit changed from its solid form into a dissolved form in a process called chemical dissolution. The result is a teasugar solution in which individual sugar molecules become uniformly distributed in the tea. But what happens if you increase the amount of sugar that you add to your tea? Does it still dissolve? In this activity you will find out how much of a compound is too much to dissolve.
BackgroundChemistry is the study of matter and how it behaves and interacts with other kinds of matter. Everything around us is made of matter, and you can explore its properties using common chemicals around your home. The way it behaves is called a property of matter. One important property is called solubility. We think about solubility when we dissolve something in water or another liquid. If a chemical is soluble in water, then the chemical will dissolve or appear to vanish when you add it to water. If it is not soluble, or insoluble, then it will not dissolve and you will still see it floating around in the liquid or at the bottom of the container.
Materials
Preparation
Procedure
More to explore
Per Quantity Of Solvent
In particular, chemical handbooks will often express the solubility of a substance in a liquid as grams of solute per of solvent or, less commonly, as grams per litre . The quantity of solvent can instead be expressed in mass, as in g/100g” or g/kg. The number may be expressed as a percentage in this case, and the abbreviation “w/w” may be used to indicate “weight per weight”.
Alternatively, the quantity of solute can be expressed in moles instead of mass if the quantity of solvent is given in kilograms, the value is the molality of the solution .
Don’t Miss: What Is L In Physics
What Is Insoluble In Science
Definition: An insoluble substance is a substance that will not dissolve in a solvent even after mixing .
What does the term insoluble mean in chemistry?
Some substances may be insoluble in some cases for example, water is insoluble in alcohol, but it is soluble in acid. The term insoluble can refer to gases, solids and liquids. The constant K_sp is used in chemistry to describe the solubility of certain types of compounds in water.
What is the definition of solubility in science?
Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. Solubility is defined as the maximum quantity of a substance that can be dissolved in another.
When does something become soluble in the presence of another substance?
When something can be dissolved or liquefied in the presence of another substance, it is considered soluble by scientific standards. The degree of solubility can vary.
Which is more soluble a solvent or a solute?
The solute is the substance that dissolves, while the solvent is the substance into which the solute dissolves. The stronger the attraction between the solute and the solvent, the greater the solubility of the substance. When the attraction is weak, there is less solubility.
What Does Insoluble Mean In Chemistry
Insoluble Substance. Q. Zhang, X. Chris Le, in Comprehensive Sampling and Sample Preparation, 2022.
- Suggested Approach for Treatment of Industrial Wastewater by Formation of an Insoluble Substance
- Industrial impacts
Extraction Techniques and Applications: Biological/Medical and Environmental/ForensicsQ. Zhang, . . . X. Chris Le, in Comprehensive Sampling and Sample Preparation, 20123. 06. 9. 1. 3 Microscopic ExaminationMicroscopic examination can detect and identify urine sediments, insoluble substances in urine. The sediments include cells , oval fat bodies, bacteria, yeast, mucus, casts and crystals. These components come from the blood, kidneys, and lower genitourinary tract. External contaminants can also contribute to sediments. Since the presence of some of these components is considered normal unless their levels are increased, specimen volume and sediment examined volume are important and should remain constant. 66The pretreatment of standardized urine sediment examination is centrifugation.
Video advice: Soluble and insoluble materials Experiment Elementary Science
This is an elementary science video for kids that help them understand and distinguish soluble and insoluble materials.
You May Like: What Does Co Stand For In Chemistry
Examples Of Insoluble In A Sentence
insolubleinsolubleinsoluble Good Housekeepinginsoluble USA TODAYinsoluble Country Livinginsoluble Country Livinginsoluble The Atlanticinsoluble WSJinsoluble SELFinsoluble Popular Mechanics
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘insoluble.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Solubility Effects On Reactions
Depending on the solubility of a solute, there are three possible results: 1) if the solution has less solute than the maximum amount that it is able to dissolve , it is a dilute solution 2) if the amount of solute is exactly the same amount as its solubility, it is saturated 3) if there is more solute than is able to be dissolved, the excess solute separates from the solution. If this separation process includes crystallization, it forms a precipitate. Precipitation lowers the concentration of the solute to the saturation in order to increase the stability of the solution.
Recommended Reading: 7 5 Practice Exponential Functions Glencoe Algebra 1
Is Sugar Soluble In Water
Water /Soluble inWater is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earths hydrosphere and the fluids of all known living organisms. It is vital for all known forms of life, even though it provides neither food, energy, nor organic micronutrients.
Wikipedia
Definition Of Insoluble Salts
Insoluble salts are ionic compounds that are insoluble in water: the salt continues to exist as a solid rather than dissolving in the liquid.On the atomic scale, the ionic lattice of an insoluble salt remains intact the ionic lattice does not break up to allow the salt ions to be surrounded by water molecules and so form a solution.
If an insoluble salt forms by the reaction of soluble substances in water and falls out of solution, we call it a precipitate .
When a salt such as sodium chloride dissolves in water, its ionic lattice is pulled apart so that the individual sodium and chloride ions go into solution.
NaCl Na+ + Cl-
In practice, many salts that are described as insoluble do actually ionize slightly in water, releasing ions into solution.
The undissolved solid in contact with water will come into equilibrium with the ions it has released. At this point the solution is said to be saturated.
In simple cases, where there are no common ions or competing equilibria, the ion concentrations depend only on the equilibrium constant for the particular salt.
When we talk about solubility equilibria we always write the equilibrium with the solid on the left.For example:
Ba2 Ba2+ + 2 IO3-
The equilibrium constant expression for an insoluble salt is written following the same rules as for any other equilibrium.The equilibrium constant is called the solubility product, Ksp.The Ksp expression for the above equilibrium is:
Ksp = 2
Note:Search the Dictionary for More Terms
Recommended Reading: What Does Factor Mean In Algebra
Factors That Affect Solubility
Whether or not a substance is soluble, and to what degree, depends on a variety of factors. Solutes typically will dissolve best in solvents that have the most molecular similarities. Polarity is a major factor in a substance’s solubility. Molecules where one end is negatively charged and the other is positively charged are considered polar, meaning that they have electrical poles. If a molecule does not have this ionic makeup, it is considered nonpolar.
Generally, solutes are soluble in solvents that are most similar to them molecularly. Polar solutes will dissolve better in polar solvents, and non-polar solutes will dissolve better in non-polar solvents. For example, sugar is a polar solute, and absorbs very well in water. However, sugar would have a low solubility in a nonpolar liquid like vegetable oil. In general, solutes will also be more soluble if the molecules in the solute are smaller than the ones in the solvent.
Other factors that affect solubility are pressure and temperature. In some solvents, when heated the molecules vibrate faster and are able to break apart the solute. Pressure is mainly a factor when a gas substance is involved, and has little to no effect on liquid substances.
Qualifiers Used To Describe Extent Of Solubility
The extent of solubility ranges widely, from infinitely soluble such as ethanol in water, to essentially insoluble, such as titanium dioxide in water. A number of other descriptive terms are also used to qualify the extent of solubility for a given application. For example, U.S. Pharmacopoeia gives the following terms, according to the mass msv of solvent required to dissolve one unit of mass msu of solute:
| Term | |
|---|---|
| 0.000245 | 409000 |
The thresholds to describe something as insoluble, or similar terms, may depend on the application. For example, one source states that substances are described as “insoluble” when their solubility is less than 0.1 g per 100 mL of solvent.
Read Also: Google Geometry Dash 2.0