S Of Finding The Equivalence Point
There are several different ways to identify the equivalence point of a titration:
Color Change – Some reactions naturally change color at the equivalence point. This may be seen in redox titration, particularly involving transition metals, where the oxidation states have different colors.
pH Indicator – A colored pH indicator may be used, which changes color according to pH. The indicator dye is added at the beginning of the titration. The color change at the endpoint is an approximation of the equivalence point.
Precipitation – If an insoluble precipitate forms as a result of the reaction, it can be used to determine the equivalence point. For example, the silver cation and chloride anion react to form silver chloride, which is insoluble in water. However, it can be difficult to determine precipitation because the particle size, color, and sedimentation rate may make it difficult to see.
Conductance – Ions affect the electrical conductivity of a solution, so when they react with each other, the conductivity changes. Conductance may be a difficult method to use, especially if other ions are present in the solution that can contribute to its conductivity. Conductance is used for some acid-base reactions.
Spectroscopy – Spectroscopy can be used to find the equivalence point if the spectrum of the reactant, product, or titrant is known. This method is used to detect etching of semiconductors.
Titration Technique Of Analytical Chemistry
Titration is a technique used in analytical chemistry to determine the concentration of unknown solutions by using solutions of known concentration. Solution of known concentration is known as titrant while the solution of unknown concentration is known as analyte in titration technique.
As we all know, the number of diabetes patients is increasing day by day worldwide. Do you know the drugs used for the treatment of diabetes contain metals in a specific amount? The metal content in a drug can be determined by titration techniques . It is a very useful, simple, and low-cost technique for various medicinal applications in the pharmaceutical field. So, you should have a good understanding of the titration technique.
To understand the titration technique, you need to have a clear understanding of the terms related to it such as a pipette, burette, titrant, analyte endpoint and equivalence point, etc. Generally, students get confused between endpoint and equivalence point so, in this article, we will discuss these two terms clearly and comparatively in detail.
Endpoint and equivalence points are closely related and confusable. Both the points show very important stages of titration during performing the titration experiment. Still, both points are different and show two different stages of titration.
Monoprotic Titration Curve Gallery
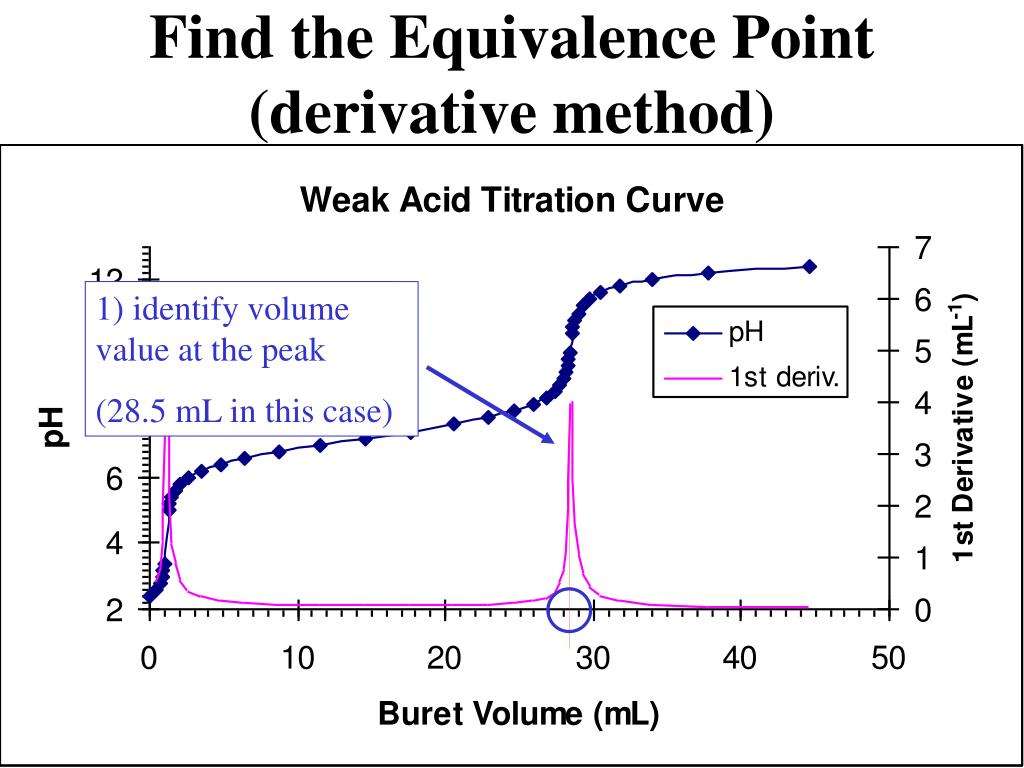
When both the titrant and sample are “strong”, we get long vertical plots at = 1. Adding even half a drop of titrant can take us across the equivalence point!
When one of the reactants is weak, the pH changes rapidly at first until buffering sets in.
In , the onset of H2O/OH- buffering near =1 makes the equivalence point more difficult to locate.
“Weak/weak” titrations tend to be problematic as the buffered regions move closer to =1. The equivalence point pH of 7 in these examples reflects the near-equality of pKa and pKb of the reactants.
Read Also: Which Is Harder Physics Or Chemistry
What Is The Definition Of Titration
Titration is defined as technique where a solution of fixed concentration is used to determine the concentration of second solution with an unknown concentration. Scientists employ titration to assess the quantity of a constituent, or analyte, of a given sample. They accomplish this by adding a known quantity of another substance, called a standard solution, to the sample.
To learn how to create a known concentration of a solution, check out this article on molarity. This standard solution contains the titrating reagent, or titrant. When titrant is added, it reacts with the analyte in a known proportion with the titrant, revealing its concentration and amount.
What Is An Equivalence Point
Pretend you are working in a forensics lab. A wealthy old woman has recently died, and the police suspect she may have been poisoned using her own heart medication. Your job is not to determine if the medicine is in her blood . Rather, your job is to figure out how much of the medicine is present. Then you will know if she took the normal amount or an overdose. To solve this mystery, you can use equivalence points. An equivalence point allows us to figure out what amount of one chemical is present when we know the amount of another chemical it reacts with.
In any chemical reaction, the equivalence point is reached when the exact amount of each chemical needed to react is present. At the equivalence point, none of the reactants are in excess – you have exactly the amount needed and no more.
An error occurred trying to load this video.
Try refreshing the page, or contact customer support.
Recommended Reading: Edgenuity Algebra 1 Answers
Equivalence Point Versus Endpoint
But how do scientists determine the point at which chemically equivalent quantities of analyte and titrant exist in the solution, so that they can quantify the analyte? They look for an equivalence point, the point at which enough titrant has combined with the analyte to neutralize it. At this specific point, the amount of titrant in the system reveals the amount of analyte in the system the moles of both species equal one another.
However, this equivalence point differs from the endpoint of a titration analysis. The endpoint indicates the end of the reaction it denotes the amount of reactant titrant needed to facilitate a complete chemical reaction with the reactant analyte. A color change in the system of interest signals that it has reached this endpoint. Materials called indicators, which undergo these color changes, can be added to the system to designate the endpoint.
Different indicators exhibit different endpoints due to their varying chemical compositions. Scientists tend to choose indicators whose endpoints roughly equal their equivalence points. When this occurs, the color transition denotes both the endpoint and the equivalence point, revealing the amount of titrant needed to equal the amount of analyte and thus the quantity of analyte in the system. Some substances, such as polyprotic acids, possess multiple equivalence points, but for a given indicator there is generally only one endpoint.
Endpoint Vs Equivalence Point: Whats The Main Difference
Endpoint and equivalence points are often confused. Both are important stages of any titration experiment and have many differences. Like other titration terms such as titrants, analyte, burette, and pipette, endpoint and equivalence points are equally important in fully understanding the titration technique.
In a nutshell,
Endpoint is the point in the titration process where the indicator changes its color whereas the equivalence point indicates the completion of the reaction between titrant and the substance being titrated .
| Prerequisites |
| Titration: the phenomenon of chemical equivalence |
| Indicators: the science behind color changes |
Also Check: What Does Percent Error Tell You
Summary Half Equivalence Point Vs Equivalence Point
Titrations are analytical techniques in chemistry that are important in determining the unknown concentrations of given samples. The key difference between half equivalence point and equivalence point is that half equivalence point is the midpoint between the starting point and equivalence point of a particular titration whereas equivalence point is where the chemical reaction ends.
Reference:
1. Titration Curves & Equivalence Point . Khan Academy, Available here.
Image Courtesy:
1. Titration of weak acid with strong base By Quantumkinetics Own work via Commons Wikimedia
S Of Determining The Equivalence Point
- Color change of self-indicators In reactions involving self-indicators as reactants, the color change indicates the equivalence point of the titration since indicators are not used.
- Endpoint Sometimes, equivalence point can be considered as the endpoint since they are approximately equal.
- Conductance Conductance can also be used to determine the equivalence point of the titration. Here, the conductance should be measured throughout the titration, and the equivalence point is where a rapid change of conductance occurs. This is a bit difficult method.
- Spectroscopy This method can be used for colorful reaction mixtures. The determination is done according to the rapid change in wavelengths that are absorbed by the sample.
Also Check: Geometry Segment Addition Postulate Worksheet Answer Key
Main Difference Equivalence Point Vs Endpoint
Titration methods are often used to identify and quantify the components in a solution mixture. Some titrations are done along with an indicator that is helpful in indicating the end of the chemical reaction. This indication is given by changing the color of the system. But some reactants act as indicators themselves. Thus, indicators are not used in all systems. The results of a titration mainly depend on the person who does the titration since different people identify the endpoint of a titration at different points. However, the end point is not the point where the reaction actually ends. The end of the reaction is given by the equivalence point. The endpoint indicates that the equivalence point has been reached. The main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical reaction ends whereas end point is the point where the color change occurs in the system.
Key Concepts And Summary
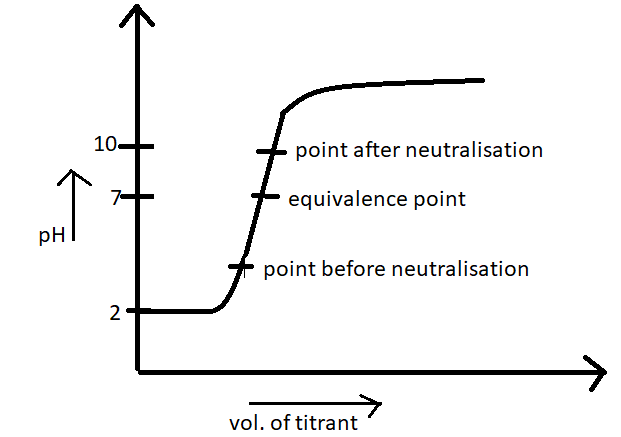
A titration curve is a graph that relates the change in pH of an acidic or basic solution to the volume of added titrant. The characteristics of the titration curve are dependent on the specific solutions being titrated. The pH of the solution at the equivalence point may be greater than, equal to, or less than 7.00. The choice of an indicator for a given titration depends on the expected pH at the equivalence point of the titration, and the range of the color change of the indicator.
Don’t Miss: Which Is Harder Chemistry Or Physics
What Is Equivalence Point
Equivalence Point is the actual point where the chemical reaction in a titration mixture ends. A titration is done often to determine the concentration of a substance in a liquid. If the substance is known, we can use a titrant with a known concentration that can react with the substance. The titrant is called a standard solution because its exact molarity is known.
For example, let us consider the reaction between NaOH and HCl. This is an acid-base reaction. We can use either NaOH or HCl as the titrant of the concentration. The titrant is placed in the burette and is added slowly to the titrand/analyte until a color change occurs in the reaction mixture. An indicator should be used as NaOH or HCl are not self-indicators. The point where a color change occurs is taken as the endpoint of the titration. But it is not the equivalence point of the reaction.
Here, the equivalence point is the point where all HCl molecules have reacted with NaOH . Here, the moles of titrant should be equal to the moles of the unknown analyte.
Figure 1: Titration curve for a titration of an acid with a base
What Is The Difference Between Equivalence And Half
I learned in class that the equivalence point is the point of neutralization where the amounts of acid and base are equivalent.
I was also told that the half-equivalence point is when the concentration of a weak acid equals concentration of conjugate base: $ = .$
I did a research and found similar definitions that dont really shed any light on the differences between them and both terms seem identical to me. What is the difference between a half-equivalence and an equivalence point, exactly?
The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. If you are titrating an acid against a base, the half equivalence point will be the point at which half the acid has been neutralised by the base. For instance, if you have 1 mole of acid and you add 0.5 mole of base, exactly half of the acid will have been neutralised. The solution remaining will be half salt and half acid.
Now I was also told that Half equivalence point is when = , the concentration of a weak acid = concentration of conjugate base.
That is correct. You make the weak acid in situ when you titrate a weak base with a strong acid, or when you titrate a weak acid with a strong base. At the half equivalence point, the pH is roughly equal to the pKa of the weak acid.
What is the difference between a half equivalence and an equivalence point?
Based on what i have been told, they sound the same to me.
Recommended Reading: Span In Linear Algebra
Which Of The Following Is/are True About Equivalence And End Points I The End Point Of A Titration Is Reached When The Titrant And Analyte Are Stoichiometrically Equal Ii When You Are Titrating A Strong Or Weak Acid With A Strong Base The End Point Will Always Be Faint Pink Using A Phenolphthalein Indicator Iii When You Are Titrating A Strong Or Weak Acid With A Strong Base The Volume Needed To Reach The End Point Will Always Be Lower Than That Of The Equivalence Point A I And Iii B Iii Only C I Ii And Iii D Ii Only
Which of the following is/are TRUE about equivalence and end points?
I. The end point of a titration is reached when the titrant and analyte are stoichiometrically equal.
II. When you are titrating a strong or weak acid with a strong base, the end point will always be faint pink using a phenolphthalein indicator.
III. When you are titrating a strong or weak acid with a strong base, the volume needed to reach the end point will always be lower than that of the equivalence point.
How Does The Endpoint Of A Titration Differ From The Equivalence Point
The endpoint of a titration is the point where the indicator just changes colour.
The equivalence point is when the ratio of the reactants is in the amounts specified by the equation.
Ideally you would want these points to coincide.
For a strong acid and a strong base such as NaOH and HCl the final solution is neutral at pH 7:
#HCl_)+NaOH_)rarrNaCl_)+H_2O_)#
Most indicators will change colour at the equivalence point so can be used in a titration.
This is not always the case though. If you neutralise a weak base with a strong acid the final solution will not be neutral e.g:
#NH_)+HCl_)rarrNH_4Cl_)+H_2O#
This is because the ammonium ion is slightly acidic:
#NH_4^rightleftharpoonsNH_)+H_)^+#
The same problem occurs when a strong base is neutralised by a weak acid. The salt produced is slightly alkaline.
This is known as “salt hydrolysis”.
You need to choose an indicator which will change colour at the equivalence point. In this case methyl orange is suitable but phenolphthalein is not.
For more details you can look up “theory of indicators”.
Read Also: June 2017 Algebra 2 Regents Answers With Work
Endpoint Vs Equivalence Point
| Point where the indicator changes colour | The point at which the titrant is chemically equivalent to the analyte in the sample |
| Comes after the equivalence point | Comes before the endpoint |
| Weak acids can have only one endpoint | Weak acids can have multiple equivalence point |
Although the endpoint is normally regarded as the equivalence point, they are not the same. But since there is only a slight difference between an equivalent point and an endpoint, it can be considered the same for laboratory purposes. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas the latter is a point where the indicator changes colour.
What Is The Difference Between Half Equivalence Point And Equivalence Point
Titrations are analytical techniques in chemistry that are important in determining the unknown concentrations of given samples. The key difference between half equivalence point and equivalence point is that half equivalence point is the midpoint between the starting point and equivalence point of a particular titration whereas equivalence point is where the chemical reaction ends.
Below tabulation summarizes the difference between half equivalence point and equivalence point.
Recommended Reading: Beth Thomas Father Jailed
Boiling Point And Water Solubility
What Is Half Equivalence Point
The half equivalence point of a titration is the halfway between the equivalence point and the starting point . The importance of this point is that at this point, the pH of the analyte solution is equal to the dissociation constant or pKa of the acid used in the titration. The half equivalence point occurs at the one-half volume of the first equivalence point of the titration. If there are multiple equivalence points in the titration, there are several half equivalence points that are equal to the number of equivalence points. For example, a second-half equivalence point occurs at the midpoint between first and second equivalence points.
Recommended Reading: Paris Jackson Biological Father Mark Lester