Sf6 Molecular Geometry Bond Angles
Valence Electrons In Sf6
We have two kinds of atoms in SF6, sulfur, and fluorine.
There is one atom of sulfur whereas there are 6 atoms of fluorine.
Let us start making the Lewis structure by knowing about the valence electrons of Sulfur and Fluorine.
Valence electrons of Sulfur = 6, and Valence electrons of Fluorine = 7
There are 6 atoms of Fluorine in this compound, so the total valence electrons of Fluorine here are = 7*6 = 42 valence electrons
Therefore, total valence electrons of SF6 is = 42+6 = 48 valence electrons
Has the question arisen in your mind that why the valence electrons in sulfur are 6?
Well, if yes then let us give you the answer to it.
The atomic number of sulfur is 16. These are the total number of electrons that are present in this element. If we start filling these electrons by the electronic configuration method then we will have the following arrangement,1s2 2s2 2p6 3s2 3p4
In the outermost shell, we have 6 electrons that are also known as valence electrons in the case of Fluorine.
How Many Electron Domains Does Xef6
seven electron pairsXeF6 has seven electron pairs. It consists of 6 bond pairs and one lone pair. Xenon has 8 electrons in its valance shell and it forms six bonds with the fluorine atoms. When the fluorides of xenon have formed the electrons in the valence shell of xenon get unpaired and are promoted to vacant 5d orbitals.
Don’t Miss: Fsa Warm Ups Grade 5 Answer Key
Molecular Geometry Of Sf4
Before going to understand the molecular geometry of SF4, let us understand What Molecular Formula is.
The molecular formula is the varieties and number of particles available in the atoms group. Here, as we are discussing SF4, the SF4 is a Molecule, and It covers an AX4E species.
The 3-dimensional arrangement of atoms or fragment which create a molecule by getting together is called Molecular Geometry. It can be specified regarding the bond lengths or bond angles. It also regulates many properties, such as:
-
Reactivity
-
Biological Activity
-
Magnetism
There are various types of Molecular structures such as linear, tetrahedral, bent, octahedral, trigonal pyramidal, trigonal planar, and more. SF4 covers under the Trigonal Bipyramidal structure because of its electron arrangements.
What Is The Electronic Configuration Of A Nh3 Atom
NH3 Hybridization The Nitrogen atom has the electronic configuration of 1s2 2s2 2px1 2py1 2pz1. When it shares the electrons with Hydrogen atoms, one s-orbital and three p-orbitals hybridize and overlaps with s orbitals of a Hydrogen atom to form sp3 hybridization. Thus, Ammonia or NH3 has sp3 hybridization.
Don’t Miss: Is Paris Jackson Michael’s Biological Child
Solved: Draw The Lewis Structure For Sf6 And Then Answer
27. The electron-domain geometry of a sulfur-centered compound is trigonal bipyramidal. The hybridization of the central nitrogen atom is _____. a. sp b. sp 2 c. sp 3 d. sp 3d e. sp d2 28. The hybridization of orbitals on the central atom in a molecule is sp. The electron-domain geometry around this central atom is _____. a. octahedral b In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Electron domains may also be called electron groups. Bond location is independent of whether the bond is a single, double, or triple bond Problem: Consider the molecule IF3.i) What is the electron domain geometry and the molecular geometry of IF 3?ii) Is IF3 polar or nonpolar? FREE Expert Solution. 99% Problem Details. Consider the molecule IF 3. PBr5, XeF2, SF6.
Chemical Bonding Flashcards Quizle
Recommended Reading: Paris Jackson Dna Test
Sulfur Hexafluoride Sf6 Molecular Geometry & Polarit
- An example of toctahedral molecular geometry that results from six electron pair geometry is SF 6. The sulfur atom has 6 valence electrons. However this is an example where six fluoride atoms are present and the octet is expanded. The Lewis diagram is as follows: F = 7 e- x 6 = 42 e-S = 6 e- = 6 e-Total = 48 e
- Thus, the SF6 electron geometry is considered to be octahedral. All the F-S-F bonds are 90 degrees, and it has no lone pairs. BrF 3 contains three bonded and two nonbonded electron domains, giving a trigonal pyramidal e-domain geometry and a T shaped molecular geometry. The bond angles are compressed relative tothose in a perfect trigonal.
- ing Electron-Group & Molecular Geometry The repulsive forces between bonding and non-bonding electrons deter
- imizes electron repulsion. Generic Formula: MX or MX 2
E represents a non-bonded electron domain . For example, methane CH 4 is an AX 4 molecule while ammonia NH 3 is an AX 3 E molecule. Both of these molecules have four electron domains and hence would have a tetrahedral domain geometry as listed above. However, the shape of the molecules are not the same as we will see below shape of sf6 according to vsepr theory February 12, 2021 / 0 Comments / in Uncategorized / by.
Why Does Oxygen Always Have 2 Lone Pairs
This method works because each covalent bond that an atom forms adds another electron to an atoms valence shell without changing its charge. The full valence shell for hydrogen is 2 and the number of electrons in bonds is also 2. The difference is zero. Oxygen typically has 4 non-bonding electrons .
Recommended Reading: Electron Dot Structure Of Ccl4
Determine The Electron Geometry And Molecular Geometry Of
Iodine heptafluoride, IF 7, is a good example of a pentagonal bipyramidal geometry. The molecule XeF 6 is an interesting case. As with IF 7, application of VSEPR rules suggests seven electron pairs. These are made up from six bonding pairs and one lone pair Hence, Electron Domain theory accounts for the geometry of PCl5. Second, SF6 is a fairly unreactive gaseous compound in which all six fluorine atoms are bonded to the central sulfur atom. Again, it is clear that the octet rule is violated by the sulfur atom, which must therefore have an expanded valence
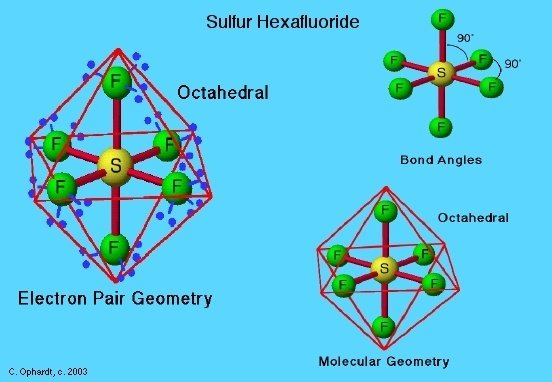
Molecular Geometry And Mo Diagram Of Sf6
There are Fluorine atoms all around Sulfur which gives the compound a kind of symmetry when we look at it on a planar level.
When we look at the molecular geometry of any compound we get to see a 3-D image of how the atoms are distributed which we cannot identify while making a Lewis Structure.
With the help of molecular geometry, we can find bond angles as well. For SF6 the bond angle is 90 Degrees as the atoms are distributed evenly around the central atom Sulfur.
You can find the shape of a compound with the help of the VSEPR theory. This theory deals with electron repulsion and compounds taking up a shape to reach stability.
Here, in SF6, there are 6 sigma bonds between Sulfur and Fluorine and 3 lone pairs on each Fluorine atom. These lone electrons repel each other and maintain symmetry around the central atom.
That is why the shape that SF6 takes is octahedral, which can be termed as its molecular geometry too.
Below is the MO diagram of SF6.
You can watch this video to learn how to make a MO diagram step-by-step.
You May Like: My.hrw Answer Key
Is Sf6 Polar Or Nonpolar
SF6 is a nonpolar molecule. This is because the VSEPR theory says that when six fluorine atoms are arranged symmetrically around the sulfur atom, the bond dipoles are cancelled. As a result, it is a nonpolar molecule.
It also has the same properties as non-polar molecules such as being non-soluble in water and being soluble in non-polar organic solvents.
Concluding Remarks
To summarize this article we can say that in the Lewis dot structure of SF6, all the valence electrons are used up which results in forming six single bonds between S-F with no lone pairs of electrons.
The hybridization of Sulphur in this molecule is sp3d2 with the bond angles of 90 degrees.
The molecular geometry of SF6 is octahedral and it is a nonpolar molecule.
About Priyanka
To read, write and know something new every day is the only way I see my day! Well, that rhymed. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. Having an MSc degree helps me explain these concepts better. I write all the blogs after thorough research, analysis and review of the topics. And if not writing you will find me reading a book in some cosy cafe!View all posts by Priyanka
Sf4 Bond Angles And Shape
The central sulfur atom forms four bonds with the neighboring fluorine atoms and has one lone pair of electrons. Fluorine atoms on the equatorial positions have the bond angles of 102 degrees, and the axial ones have 173 degrees, which are a little different than the trigonal bipyramidal molecular geometry leading to a see-saw shape.
The lone pair on the central atom leads to the change in the bond angles from 120 degrees to 102 degrees for equatorial fluorine atoms and 173 degrees instead of 180 degrees for axial fluorine atoms.
Concluding Remarks
To conclude all the properties we can say that,
- Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure.
- There are three lone pairs on each fluorine atom.
- It has a molecular geometry of the formula AX4E it forms a see-saw shape and has a trigonal bipyramidal molecular geometry.
- SF4 has sp3d hybridization and is polar in nature.
About Priyanka
To read, write and know something new every day is the only way I see my day! Well, that rhymed. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. Having an MSc degree helps me explain these concepts better. I write all the blogs after thorough research, analysis and review of the topics. And if not writing you will find me reading a book in some cosy cafe!View all posts by Priyanka
Don’t Miss: Mcdougal Geometry Workbook Answers
C2h6 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram
C2H6, known as ethane, is a saturated open-chain hydrocarbon or we can say that it comes under the alkane family. Hydrocarbon is an organic compound, which contains only carbon and hydrogen. Saturated hydrocarbons are those hydrocarbons, which contain carbon-hydrogen and carbon-carbon single bonds.
Saturated hydrocarbons are further classified into alkane and cycloalkane . Ethane can also be written as CH3-CH3.
Ethane is colorless and odorless gas at standard temperature and pressure. The melting and boiling point of ethane are -182.8 °C and -89 °C, respectively. The flashpoint of ethane is -135 °C and hence, its vapors ignite easily by an ignition source.
The molar mass of ethane is 30.07 g/mol. Ethane is obtained from natural gas and petroleum industrially. It can also be prepared from ethene, ethyl chloride, and sodium acetate in the laboratory. Here are some methods of preparation of ethane:
CH2 = CH2 + H2 Pt/Pd/Ni> CH3 CH3
CH3 CH2Cl + H2 Zn/H+ > CH3 CH3 + HCl
2CH3Cl + 2Na Dry ether> CH3 CH3 + 2 NaCl
2 CH3COONa + 2H2O Electrolysis> CH3 CH3 + 2NaOH + H2 + 2CO2
Let us discuss the basic concepts of ethane such as its Lewis structure, polarity, hybridization of carbon atom in ethane, and its Molecular orbital diagram to understand its chemical bonding in terms of molecular orbitals.
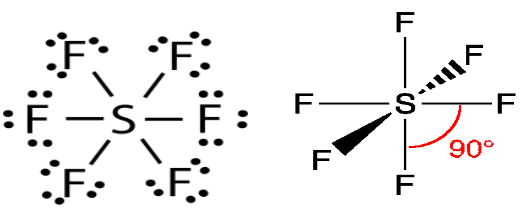
Lewis Dot Structure Of Sf6
The central atom here is Sulfur as it is less electronegative than Fluorine. This is because the outer shell of Fluorine has 5 electrons and it needs one more electron to reach stability, which is easier to attain.
As there are 6 atoms of Fluorine, there will be a formation of 6 bonds between Sulfur and Fluorine. Sulfur will share 6 electrons with one electron of each Fluorine atom.
Here is a pictorial representation of the same.
A bond formation is shown by a single straight line and lone pairs of electrons are shown by two dots for each pair.Now when we know that how many bonds are getting formed, let us see how many lone pairs will be made.
When 6 bonds are formed, 12 electrons will be used and that leaves us with 36 valence electrons.
Did you see that there are more than 8 electrons in the outer shell of Sulfur?
The reason for this is that some atoms can expand their valence shells. When the outermost shell can accommodate more electrons these kinds of conditions occur.
Now, the remaining electrons will be lone pairs of electrons on the Fluorine atom. On every Fluorine atom, there are going to be 3 lone pairs of electrons.
So there will be 18 lone pairs of electrons, with 36 electrons in total.
The octet of all the Fluorine atoms is complete and as Sulfur is an exception its octet is expanded.
This is all about the Lewis Structure of SF6, now let us look at the hybridization and molecular geometry of the compound.
Also Check: How To Find Delta Math Answers With Inspect Element
Sf6 Molecular Geometry Lewis Structure Shape And Polarity
Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among chemistry enthusiasts.
Henri Moissan discovered the existence of SF6. Incidentally, he is also the discoverer of fluorine. The standard way of synthesizing SF6 is to expose S8 to F2. This method causes the formation of a few sulfur fluorides, but those can be eliminated through heating and then using NaOH to remove any additional SF4 molecules.
SF6 cannot be used immediately after synthesis. It needs to be purified to get rid of all reactive fluorides. After that, it needs to go through pyrolysis.
Here in this blog post, we will learn the Lewis Structure of SF6 and its Bond angles, Molecular geometry and shape that can help us understand the physical properties of this molecule.
| Name of molecule | |
| No of Valence Electrons in the molecule | 48 |
What Is The Hybridization Of Sf4
SF4 has only one lone pair and four sigma bonds of F. The central atom is S. So, to explain in simple terms, its bonding regions are four having one lone pair.
There are 34 valence electrons and 5 electron pairs. These five valence atomic orbitals present on the middle atom S are hybridized to resultantly form five sp3d hybrid orbitals. There are four of the hybrid orbitals overlapped with 2P-orbitals. After this complete process, the last hybrid orbital holds a lone pair.
During SF4 formation, the sulfur atom will produce bonds with each of fluorine atoms where 8 of valence electrons are used. Besides, the 4 fluorine atoms will have 3 lone pairs of electrons in its octet, which will utilize 24 valence electrons further. Besides, two electrons will be placed as a lone pair in the sulfur atom. Now, we can determine the hybridization of Sulfur by considering the number of regions of electron density. When bonding occurs, there is a formation of 4 single bonds in Sulfur, and it has only 1 lone pair. By this, we can say that the number of electron density regions is 5.
The S atom in the middle containing the 5 valence atomic orbitals is hybridized to form five sp3d hybrid orbitals. In the 2P-orbitals, 4 hybrid orbitals are overlapped, and the fifth orbital has a lone pair. It will also help in determining the hybrid orbitals count used by the atom by knowing the steric number. Sulfur will use 5 orbitals, including 1 3s-orbital, 3 3p-orbitals, and 1 3d-orbital.
Also Check: Beth Thomas Brother Now