Van Der Waals Interactions
Like hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. They occur between polar, covalently bound, atoms in different molecules. Some of these weak attractions are caused by temporary partial charges formed when electrons move around a nucleus. These weak interactions between molecules are important in biological systems.
Name The Forces That Keep Reacting Atoms Together
In metals, outer orbitals of atoms overlap and so the electrons present in them do not belong to any particular atom but flow over to all atoms as well and bind them all together . Atoms that have to lose and gain electrons, becomes ions and are held together by the electrostatic forces of attraction . When atoms equally give and share electrons, the shared electrons becomes the unifying force between them . Electron-deficient and free lone pair containing molecules may again and satisfy the octet thirst of the electron-deficient atom. The shared electron bridges the electron-rich atom with electron-deficient atom .
Definition: What Is A Chemical Bond
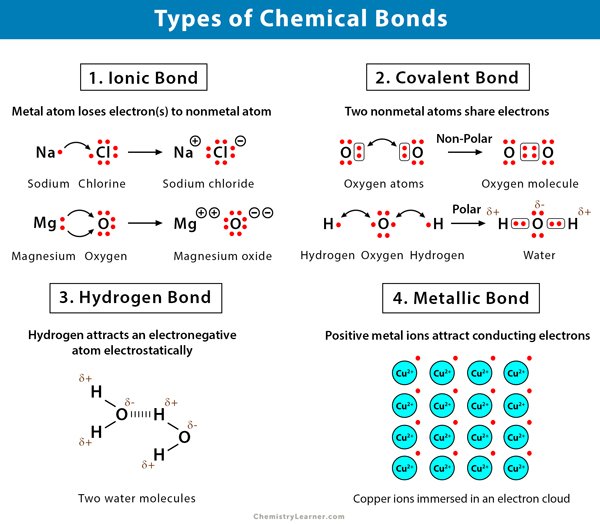
Chemical bonds are forces that hold the atoms together in a molecule. They are a result of strong intramolecular interactions among the atoms of a molecule. The valance electrons of the atoms participate in chemical bonds. When two atoms approach each other, these outer electrons start to interact. Although electrons repel each other, they are attracted to the protons within atoms. The interplay of forces results in the formation of bonds between the atoms. The main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond.
A bond between two atoms depends upon the electronegativity difference between the atoms. If the electronegativity difference is significantly high, the atoms transfer electrons to form ions and thereby form an ionic bond. If the electronegativity difference is zero or small, then the atoms combine to form covalent bonds.
You May Like: How To Calculate Ihd Organic Chemistry
Bonds Stability And Compounds
Covalent interactions are directional and depend on orbital overlap, while ionic interactions have no particular directionality. Each of these interactions allows the atoms involved to gain eight electrons in their valence shell, satisfying the octet rule and making the atoms more stable.
These atomic properties help describe the macroscopic properties of compounds. For example, smaller covalent compounds that are held together by weaker bonds are frequently soft and malleable. On the other hand, longer-range covalent interactions can be quite strong, making their compounds very durable. Ionic compounds, though composed of strong bonding interactions, tend to form brittle crystalline lattices.
Forming An Ionic Bond
Once the oppositely charged ions form, they are attracted by their positive and negative charges and form an ionic compound. Ionic bonds are also formed when there is a large electronegativity difference between two atoms. This difference causes an unequal sharing of electrons such that one atom completely loses one or more electrons and the other atom gains one or more electrons, such as in the creation of an ionic bond between a metal atom and a nonmetal .
Formation of sodium fluoride: The transfer of electrons and subsequent attraction of oppositely charged ions.
You May Like: Unit 1 Geometry Basics Worksheet Answers
Seven Different Types Of Chemical Bonds And Why They Are Important
There are currently 118 different elements on the periodic table. Without chemical bonds there would only be 118 different materials in the world. There are seven kinds of chemical bonds that have been identified so far by the scientific community. Each of these bonds creates compounds that have specific characteristics and differences in strengths. These compounds each have their own uses and characteristics, but without chemical bonding they could not exist.
The chemical bonds are:
The most important of these are the ionic, covalent and metallic bonds. In each of these bonds electrons are shared between elements. Electrons orbit atoms in layers or shells according to the type of element. Different types of bonds share these electrons in a different way.
Determining The Formula Of An Ionic Compound
To determine the chemical formulas of ionic compounds, the following two conditions must be satisfied:
Magnesium and fluorine combine to form an ionic compound. What is the formula for the compound?
Mg most commonly forms a 2+ ion. This is because Mg has two valence electrons and it would like to get rid of those two ions to obey the octet rule. Fluorine has seven valence electrons and usually forms the F ion because it gains one electron to satisfy the octet rule. When Mg2+ and F combine to form an ionic compound, their charges must cancel out. Therefore, one Mg2+ needs two F ions to neutralize the charge. The 2+ of the Mg is balanced by having two -1 charged ions. Therefore, the formula of the compound is MgF2. The subscript two indicates that there are two fluorines that are ionically bonded to magnesium.
On the macroscopic scale, ionic compounds form crystalline lattice structures that are characterized by high melting and boiling points and good electrical conductivity when melted or solubilized.
Also Check: Example Of Span Linear Algebra
The Oxygen Molecule Is Paramagnetic Is There An Explanation
Oxygen atom shares two electrons, each with another oxygen atom to form the oxygen molecule. Oxygen molecule exhibits paramagnetic nature indicating unpaired electrons. A molecular orbital theory has been proposed to explain this. According to this theory, atoms lose their orbitals and rather form an equal number of orbital covering the entire molecule and hence the name molecular orbital. Filling up of these orbitals in increasing energy order leaves unpaired electron explaining the paramagnetic behaviour of oxygen molecule.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Overview Of Main Types Of Chemical Bonds
A chemical bond is an attraction between atoms. This attraction may be seen as the result of different behaviors of the outermost or valence electrons of atoms. These behaviors merge into each other seamlessly in various circumstances, so that there is no clear line to be drawn between them. However it remains useful and customary to differentiate between different types of bond, which result in different properties of condensed matter.
In the simplest view of a covalent bond, one or more electrons are drawn into the space between the two atomic nuclei. Energy is released by bond formation. This is not as a result of reduction in potential energy, because the attraction of the two electrons to the two protons is offset by the electron-electron and proton-proton repulsions. Instead, the release of energy arises from the reduction in kinetic energy due to the electrons being in a more spatially distributed orbital compared with each electron being confined closer to its respective nucleus. These bonds exist between two particular identifiable atoms and have a direction in space, allowing them to be shown as single connecting lines between atoms in drawings, or modeled as sticks between spheres in models.
Read Also: Fsa Algebra 1 Eoc Practice Test Answers
What Is Covalent Bond And Its Types
Covalent bonds can be single, double, and triple bonds. Single bonds occur when two electrons are shared and are composed of one sigma bond between the two atoms. Double bonds occur when four electrons are shared between the two atoms and consist of one sigma bond and one pi bond.
List The Three Types Of Chemical Bonds And Explain The Differences Among Them
1:ionic bonding:It is done between metal and non metal.The metal loses and electron and gives it to the nonmetal.For example Na+ and Cl- so they make NaCl
2:Covalent Bonding:It is done between 2 non metals and they both share electrons for example H+ and OH- and they make H2O
3:Metallic Bonding:It is the strong attraction between closely packed positive metal ions and a ‘sea’ of delocalised electrons.An example is any metal like Zinc Iron etc
Covalents share electrons, ions give them and stick like magnets, and metals form a sea of electrons.
Explanation:
Chemical bonds are what stick atoms together. Atoms bond so that their outer shells are full of electrons and they become stable, like noble gases. Shells are what electrons organise themselves into around the nucleus – the inside shell can have 2 electrons, and after that they hold 8, excluding transition metals and really big atoms.
Covalent bonds are where two atoms electrons. The orbitals that electrons sit in overlap between one atom and the next, which satisfies both of them and makes them stable. The two atoms then can’t easily move away from each other – they are like blood brothers.
Ionic bonds are more like blood donations. A metal atom gives electrons to a non-metal atom. Electrons are negatively charged, which means the non-metal becomes more negative and the metal becomes more positive. Like North and South on a magnet, the two ions then stick together.
Don’t Miss: What Does K Stand For In Math
Other Types Of Chemical Bonds
Van der Waals Bond
Neutral molecules are held together by weak electric forces known as Van der Waals forces. Van der Waals force is a general term used to define the attraction of intermolecular forces between molecules. This type of chemical bond is the weakest of all bonds .
Examples include hydrogen bonding, dispersion forces, and dipole-dipole forces.
Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Peptide bonds are formed by a biochemical reaction that extracts a water molecule as it joins the amino group of one amino acid to the carboxyl group of neighboring amino acids. Aside from peptide bonds, hydrogen bonds, ionic bonds, and disulfide bonds are also common in proteins .
Examples include polypeptides like insulin and growth hormone.
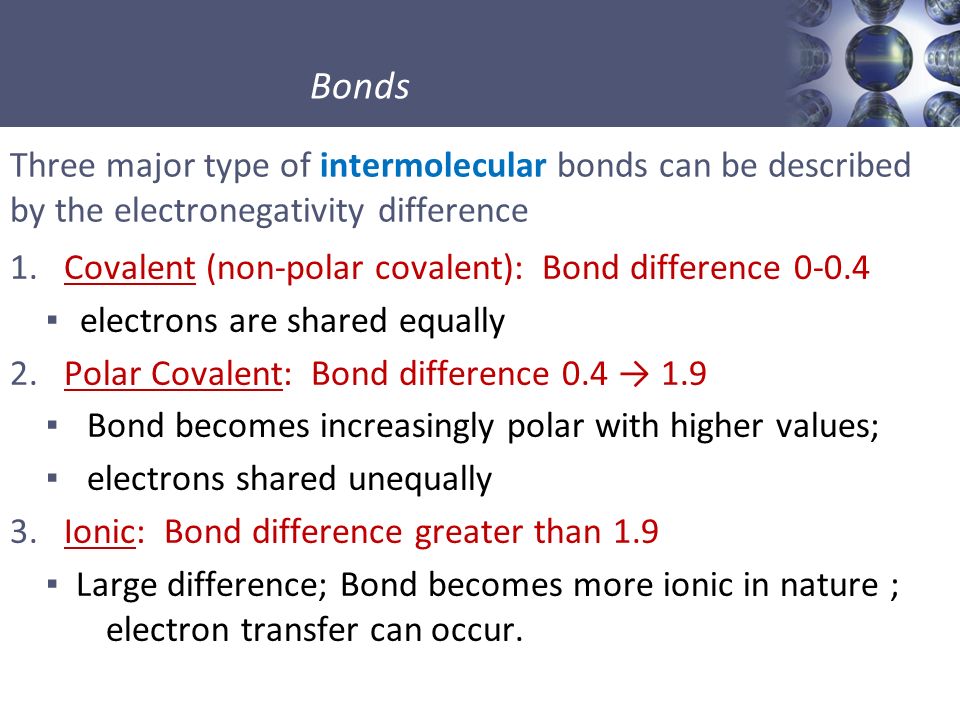
Predict Type Of Chemical Bond Based On Electronegativity
If the electronegativity values of two atoms are similar:
- Metallic bonds form between two metal atoms.
- Covalent bonds form between two non-metal atoms. Nonpolar covalent bonds form when the electronegativity values are very similar, while polar covalent bonds form when the electronegativity values are a little further apart.
If the electronegativity values of two atoms are different, ionic bonds are formed.
Also Check: Which Founding Contributors To Psychology Helped Develop Behaviorism
Covalent And Coordinate Covalent Bonds In Protein Structure
Covalent bonds involve the equal sharing of an electron pair by two atoms. Examples of important covalent bonds are peptide and disulfide bonds between amino acids, and CC, CO, and CN bonds within amino acids.
Coordinate covalent bonds involve the unequal sharing of an electron pair by two atoms, with both electrons coming from the same atom. The electron pair donor is the ligand, or Lewis base, whereas the acceptor is the central atom , or Lewis acid. These bonds are important in all interactions between transition metals and organic ligands .
Is Intermolecular Hydrogen Bonding
Two types of hydrogen bonding have been recognized: Intermolecular and intramolecular hydrogen bonding. For a substance to be soluble in water, it should be able to make a hydrogen bond with water. Any molecule which has a hydrogen atom attached directly to an oxygen or nitrogen is capable of hydrogen bonding.
Recommended Reading: How To Find Ksp Chemistry
Sp3 And Dsp2 Are Four Hybridized Orbitals But One Is The Tetrahedral Shape And Other Square Planar Why
sp3 orbitals are formed from the s -subshell with uniform electron distribution around the nucleus and of p-subshell with distribution in the three vertical axis. Hybridized orbitals, hence have their electron distribution in three dimensions, as tetrahedral directions.
In dsp2 all the orbitals involved I hybridization have their electron distribution around the same plane. Hence, the hybridized orbitals also are in the same plane giving rise to square planar geometry.
Explanation Of Kossel Lewis Approach
In 1916 Kossel and Lewis succeeded in giving a successful explanation based upon the concept of an electronic configuration of noble gases about why atoms combine to form molecules. Atoms of noble gases have little or no tendency to combine with each other or with atoms of other elements. This means that these atoms must be having stable electronic configurations.
Due to the stable configuration, the noble gas atoms neither have any tendency to gain or lose electrons and, therefore, their combining capacity or valency is zero. They are so inert that they even do not form diatomic molecules and exist as monoatomic gaseous atoms.
Don’t Miss: Countdown To The Algebra 1 Eoc Answer Key
Selective And Effective: Current Progress In Computational Structure
Giulia Bianco, … Stefano Forli, inTrends in Pharmacological Sciences, 2020
Targeted covalent inhibitors are currently showing great promise for systems that are normally difficult to target with small molecule therapies. This renewed interest has spurred the refinement of existing computational methods as well as the design of new ones, expanding the toolbox for discovery and optimization of selective and effective covalent inhibitors. Commonly applied approaches are covalent docking methods that predict the conformation of the covalent complex with known residues. More recently, a new predictive method, reactive docking, was developed, building on the growing corpus of data generated by large proteomics experiments. This method was successfully used in several inverse drug discovery programs that use high-throughput techniques to isolate effective compounds based on screening of entire compound libraries based on desired phenotypes.
N.V. Bhagavan, Chung-Eun Ha, in, 2015
Resonance In Chemical Bonding
There are molecules and ions for which drawing a single Lewis structure is not possible. For example, we can write two structures of O3.
In the oxygen-oxygen bond on the left is a double bond and the oxygen-oxygen bond on the right is a single bond. In B the situation is just the opposite. The experiment shows, however, that the two bonds are identical.
Therefore neither structure A nor B can be correct. One of the bonding pairs in ozone is spread over the region of all three atoms rather than localized on a particular oxygen-oxygen bond. This delocalized bonding is a type of chemical bonding in which bonding pair of electrons are spread over a number of atoms rather than localized between two.
Structures and are called resonating or canonical structures and is the resonance hybrid. This phenomenon is called resonance, a situation in which more than one canonical structure can be written for a species. The chemical activity of an atom is determined by the number of electrons in its valence shell. With the help of the concept of chemical bonding, one can define the structure of a compound and is used in many industries for manufacturing products in which the true structure cannot be written at all.
Some other examples:
- Carbon-oxygen bond lengths in carboxylate ion are equal due to resonance.
- Benzene
- Vinyl Chloride
The difference in the energies of the canonical forms and resonance hybrid is called resonance stabilization energy.
Don’t Miss: What Does Abiotic Mean In Biology
Polar Covalent Bonds And Hydrogen Bonds
A covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons. One electron is provided by each atom, and the pair of electrons is attracted to the positive nuclei of both atoms. The water molecule represented in Figure \ contains polar covalent bonds.
The attractive force between water molecules is a dipole interaction. The hydrogen atoms are bound to the highly electronegative oxygen atom ).
A hydrogen bond is an intermolecular and intramolecular attractive force in which a hydrogen atom that is covalently bonded to a highly electronegative atom is attracted to a lone pair of electrons on an atom or a partially negative atom in a neighboring polar molecule. Hydrogen bonds are also found intramolecularly in the tertiary and quaternary structures of protein and DNA strands.
Hydrogen bonding occurs only in molecules where hydrogen is covalently bonded to one of three elements: fluorine, oxygen, or nitrogen. These three elements are so electronegative that they withdraw the majority of the electron density in the covalent bond with hydrogen, leaving the H atom very electron-deficient. The H atom nearly acts as a bare proton, leaving it very attracted to lone pair electrons on a nearby atom.
Theories Of Chemical Bonding
In the limit of “pure” ionic bonding, electrons are perfectly localized on one of the two atoms in the bond. Such bonds can be understood by classical physics. The forces between the atoms are characterized by isotropic continuum electrostatic potentials. Their magnitude is in simple proportion to the charge difference.
Unlike the spherically symmetrical Coulombic forces in pure ionic bonds, covalent bonds are generally directed and anisotropic. These are often classified based on their symmetry with respect to a molecular plane as sigma bonds and pi bonds. In the general case, atoms form bonds that are intermediate between ionic and covalent, depending on the relative electronegativity of the atoms involved. Bonds of this type are known as polar covalent bonds.
Recommended Reading: The Segment Addition Postulate Answers
Forces Electrons And Bonds
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Atoms are the basic building blocks of all types of matter. Atoms link to other atoms through chemicals bonds resulting from the strong attractive forces that exist between the atoms.
A chemical bond is a region that forms when electrons from different atoms interact with each other. The electrons that participate in chemical bonds are the valence electrons, which are the electrons found in an atom’s outermost shell. When two atoms approach each other these outer electrons interact. Electrons repel each other, yet they are attracted to the protons within atoms. The interplay of forces results in some atoms forming bonds with each other and sticking together.