D Electrophilic Addition Of Cl2 Or Br2
Direct addition of the elemental halogen in the absence of light, often in an inert solvent such as CCl4. Although XX has no permanent dipole, the electron-rich alkene induces a + in the nearer halogen that then attaches first.
Unlike the mechanisms in C above, the first halogen that adds uses its lone pair to form a ring cation called a chloronium or bromonium ion. This is equivalent to a combined electrophilic addition and coordination step in one. The halonium ion blocks syn attack, and forces the other halogen to attack from the other side and give an anti product.
- Only the anti product is formed.
- Reaction fails for I2 or F2.
- Other mechanisms such as epoxidation, halohydrin formation and oxymercuration are somewhat similar.
Extra Topics On Nucleophilic Substitution Reaction
Our discussions so far focus on the fundamental concepts about SN1 and SN2 mechanism, and the reactions we learned about proceed in the regular way. There are some other conditions can be added to the basic nucleophilic substitution reactions, to make the reaction look different, or more challenge. However, understanding the basic concepts well is very helpful for us to deal with various situations. The reaction may looks different, but essentially it is still the same.
7.6.1 SN1 Reaction with Carbocation Rearrangement
Lets take a look at a SN1 reaction.
With the secondary substrate and neutral nucleophile , this is a SN1 reaction, and solvolysis that CH3COOH acts as both solvent and nucleophile. It is supposed to give the acetate as product, with the acetate replace the Br. However, as shown in the reaction equation that the acetate was not introduced on the carbon with leaving group Br, but was connected on the next carbon instead. What is the reason for the unexpected structure of the product?
For reactions involve carbocation intermediate, it is a common phenomena that the carbocation might rearrange, if such rearrangement leads to a more stable carbocation, and this is called carbocation rearrangement. Because of the carbocation rearrangement, the product of the above reaction is different than expected. This can be explained with the step-by-step mechanism below.
7.6.2 Intramolecular Nucleophilic Substitution Reaction
Example:
What Is The Role And Mechanism Of Action Of Tosyl Chloride
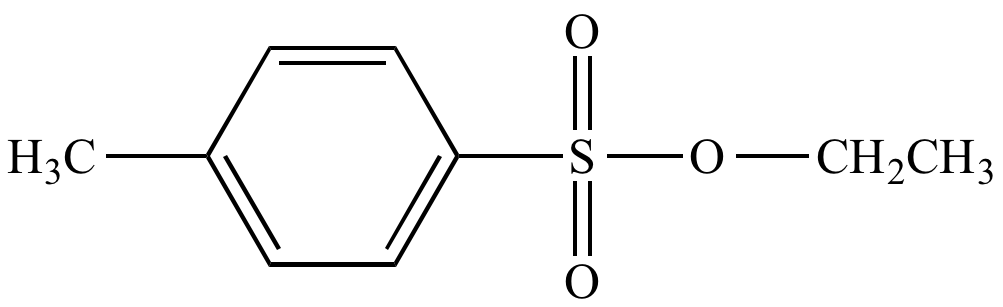
Tosyl chloride is usually used as an activating group for primary alcohols. Due to its relatively large volume and the lower reactivity of secondary and tertiary alcohols, it usually doesnt come into them, being selective to primary alcohols in most of the cases. The -OTs group formed is a good leaving group for substitution reactions.
Recommended Reading: How Did The Geography Of Greece Affect Its Development
How To Convert The Oh Into A Leaving Group
In many cases, when students are asked to convert an alcohol to an alkyl halide, they would use the corresponding halide and show the reaction with an SN2 mechanism:The result is getting no points or partial credit if you get lucky. The reason for this is because the hydroxyl ion is a poor leaving group which, in other words, means it is very reactive and even if it was to form, it would react with the other product of the reaction and shift the equilibrium back to the original state.This would also be true for a hypothetical SN1 mechanism even if we have a 3° alcohol.
The bottom line: OH cannot depart by itself and cannot be kicked out by a nucleophile.
In order to get rid of the OH group, you need to convert it to a good leaving group! There are two main approaches for this:
1) Treat the alcohol with HCl, HBr, or HI and covert the OH group to H2O+ which is an excellent leaving group and can undergo an SN1 or SN2 substitution by the halide ion .2) Activate the OH group with sulfonyl chlorides such as p-Toluenesulfonyl chloride, Methanesulfonyl chloride andTrifluoromethanesulfonyl chloride by converting it to a Tosylate , Mesylate and Triflate respectively and react it further with a nucleophile. Or, treat the alcohol with Thionyl chloride , Phosphorus tribromide , ZnCl2 etc. to convert to the corresponding alkyl halide.
Four Specific Examples Of Tosylates And Mesylates In Action
Lets finish up by seeing some specific examples of Ts and Ms in action. As they contain a good leaving group, alkyl tosylates or mesylates can perform all of the substitution and elimination reactions of alkyl halides. A few examples are shown below. Get familiar with MsCl and TsCl in both their abbreviated and expanded forms.
You May Like: Mcdougal Littell Geometry Book Answers
What Is Ots In Chemistry
Nashik, India
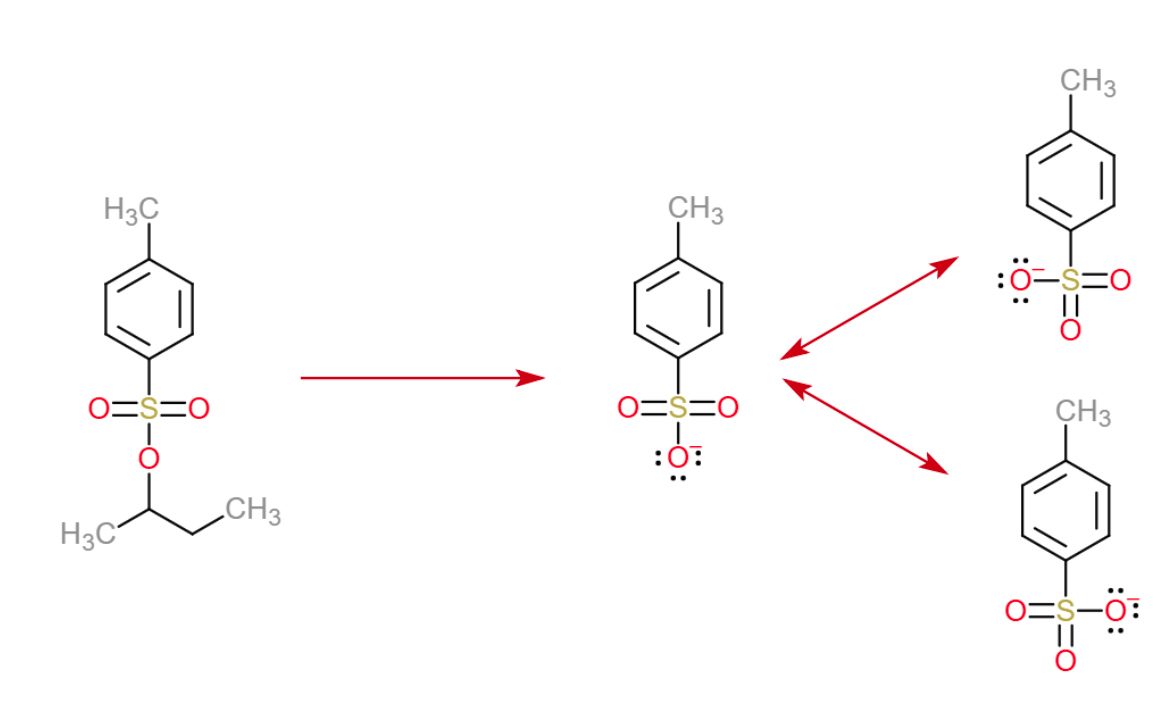
OTs in organic chemistry stands for tosylate. These are used to turn an alcohol or amine into a good leaving group. OTs in organic chemistry stands for tosylate. One way to convert alcohols to good leaving groups is to convert to a sulfonate ester like OTs or OMs . Tosylate groups are good leaving groups because their conjugate base forms are quite stabilized by resonance:. Hybridization of Atomic Orbitals, Sigma and Pi Bonds, Sp Sp2 Sp3, Organic Chemistry, Bonding.
Which photograph you have taken and astonished after looking into it?
I am not a professional photographer. I do not have a DSLR Camera like most professional photographer have. All I do is capture with my cell phone camera. Never bothered about framing or what ever grammar of photography. Recently I took a picture and to my astonishment who ever saw that picture said “Wow”. Trust me guys I have no clue why people admire this picture so much. I guess the innocent eyes of this kid flooded with curiosity draws some kind of story among viewer. Any ways I …
What is fajans rule in chemistry?
What is thf used for in organic chemistry?
What is suspension in chemistry?
How do u balance chemistry equations?
What Do You Need To Know About Ots Holdings
Here are some key highlights about the OTS Holdings IPO: OTS, which was founded in 1993, is a food manufacturing group that sells both non-halal and halal products under six house brands. Its food products have been marketed and sold in more than 25 countries, including Brunei, Hong Kong, Myanmar, India and as far as the European Union.
Read Also: Houghton Mifflin Geometry Worksheet Answers
In Conjugate Base Eliminations
The requirement for a good leaving group is relaxed in conjugate base elimination reactions. These reactions include loss of a leaving group in the position of an enolate as well as the regeneration of a carbonyl group from the tetrahedral intermediate in nucleophilic acyl substitution. Under forcing conditions, even amides can be made to undergo basic hydrolysis, a process that involves the expulsion of an extremely poor leaving group, R2N. Even more dramatic, decarboxylation of benzoate anions can occur by heating with copper or Cu2O, involving the loss of an aryl anion. This reaction is facilitated by the fact that the leaving group is most likely an arylcopper compound rather than the much more basic alkali metal salt.
Tosylateanother Good Leaving Group
- Page ID
- 57488
No headers
Alternatively, we can transform an alcohol group into sulfonic ester using para-toluene sulfonyl chloride or methanesulfonyl chloride , creating what is termed an organic tosylate or mesylate:
Again, youll have a chance to work a mechanism for tosylate and mesylate formation in the chapter 12 problems. Notice, though, that unlike the halogenation reactions above, conversion of an alcohol to a tosylate or mesylate proceeds with retention of configuration at the electrophilic carbon.
Chlorides, bromides, and tosylate / mesylate groups are excellent leaving groups in nucleophilic substitution reactions, due to resonance delocalization of the developing negative charge on the leaving oxygen.
The laboratory synthesis of isopentenyl diphosphate – the ‘building block’ molecule used by nature for the construction of isoprenoid molecules such as cholesterol and b-carotene – was accomplished by first converting the alcohol into an organic tosylate , then displacing the tosylate group with an inorganic pyrophosphate nucleophile .
| Example |
- This page has no tags.
Also Check: Geometry Dash Sneak Peek 2.0
Sn: With Tertiary And Secondary Rx + Weak Nucleophile
Usually an uncharged nucleophile such as H2O, ROH, H2S, RSH, but nonbasic negative nucleophiles such as Cl¯, Br¯, I¯ also work. With uncharged nucleophiles, there will be an acidbase step at the end to lose H+ and give an uncharged final product.
Note: If ¯OH or ¯OR are used instead, E2 elimination will dominate over SN1.
Two steps for substitution , then an acid-base step.
Favored by:
- Polar protic solvents often use the nucleophile as the solvent.
- Cold temperatures reduces chances of E1 elimination
- Tertiary & secondary R. Never primary unless resonance stabilizing R+
Examples of nucleophilic substitution
In the SN2 example, note the primary alkyl group, the strong nucleophile and the polar aprotic solvent all point to SN2 as the mechanism.
In the SN1 example, note the resonance-stabilized secondary carbocation, and the weak nucleophile , which also serves as the polar protic solvent. R, Nuc and solvent all point to SN1 as the mechanism.
E2 Elimination: Rx + Strong Base
- Shown above with ¯OH as base, but ¯OR works similarly.
- Works with most alkyl halides, tertiary is easiest but others work fine.
- However, there must be an H anti to the X on the neighboring carbon .
- This anti constraint means that only one stereoisomer of the product is formed, alkene 1.
- Favored by heat.
- Zaitsevs Rule applies with most bases, unless they are sterically hindered . Common bases include KOH , NaOCH3, NaOEt, KOtBu and some amine bases.
Recommended Reading: Geometry Dash 1 20
Organic Chemistry Is A Branch Of Chemistry That Focuses On Compounds That Contain Carbon
An answer to the question of what exactly is organic chemistry cannot be sufficiently provided without understanding the mechanisms by which organic chemists. Since fossil fuels are nonrenewable resources, it is. Organic chemists have developed a huge array of chemical reactions that can convert one organic compound to another. An alkyl group is a radical with a certain number of carbons and is of the general formula. What is organic chemistry and what is included within this science subject area ? What is the proper name for this molecule? So, using this reagent here tosyl chloride is used in almost equimolar ratios to that of aniline. Current trends in organic chemistry are. All living organisms are composed of organic compounds, as are most foods, medicines, clothing fibers, and plastics. The clothes you wear are. An introduction to organic chemistry: I’ve been told that $\ce$ is a good leaving group for substitution reactions, but i’m not sure how it works or why it is a good leaving group. What does | mean in chemistry?
The following list of topics in organic chemistry includes key material covered in introductory courses but is not specific to any particular syllabus
All living organisms are composed of organic compounds, as are most foods, medicines, clothing fibers, and plastics.
Making Alcohols Into Good Leaving Groups Part 2
Weve seen that alcohols are poor substrates for substitution reactions. The main problem is that the hydroxyl group is a strong base, and thus a poor leaving group.
In the last post we saw that we can convert alcohols into alkyl halides by adding strong hydrohalic acids : the strong acid protonates R-OH to give R-OH2+ and then substitution by the halide ion can occur.
In contrast to alcohols, alkyl halides are GREAT substrates for nucleophilic substitution reactions.
Unfortunately this doesnt always work well. There are two main problems. First of all, on certain secondary alcohols the reaction proceeds through an SN1 pathway, which can lead to rearrangements. Secondly, in so doing, we can end up scrambling any stereocenters that are present. For instance if we start with one enantiomer in the reaction below, we end up with some racemization of the final product.
So is there some other way to convert alcohols into good leaving groups that doesnt have these problems?
Sure thing!
Recommended Reading: Punchline Bridge To Algebra Integers And Expressions Answer Key
C Addition Of Hx Or H2o To Alkenes
Shown with propene as an example. Follows Markovnikovs Rule: The H goes onto the less substituted carbon, while the X or OH goes onto the more substituted carbon. Mechanisms involving alkynes are quite similar, though in that case H2O addition does involve a subsequent tautomerization step. Note that the pi bond disappears, and two new sigma bonds are formed . The added H has not been shown explicitly in this first scheme.
What Is Ots In Organic Chemistry
What Is Ots In Organic Chemistry. An organic chemist is a chemist with a college degree in chemistry. Organic chemistry is the study of the structure, properties, and reactions of organic compounds and organic materials.
Current trends in organic chemistry are. What is the proper name for this molecule? Jump to navigation jump to search. The substances contained in the subject can have hydrogen, oxygen, nitrogen, sulfur, the halogens and a variety of other molecules contained in their molecular formulae. Most organic chemicals are covalent compounds, which is why we introduce organic chemistry here.
Recommended Reading: Percent Difference In Physics
Approach : Conversion Of Alcohols To Alkyl Halides With Hx
Both, the substrate and the acid affect the conversion in the following way:
1) The strength/reactivity of the acid, hence the efficiency of the conversion is increasing in the following order:
HCl < HBr < HI
2) More substituted alcohols usually react more rapidly with HX:
Methyl and primary alcohols are converted to alkyl halides via SN2 reaction according to the general mechanism shown below. The I and Br are strong enough nucleophiles to attack the primary carbon and the +OH2, in turn, is an excellent leaving group in form of neutral water molecule. HCl is the weakest acid among these and Cl is not a strong nucleophile so the reaction with HCl is not as efficient and sometimes requires an additional catalyst like ZnCl2. Check with your instructor for more details.
Secondary alcohols can undergo SN2 and SN1 reactions with SN1 being the predominant pathway, so if you want to have control over the stereochemistry of the reaction, the second approach, which we will discuss in more detail below, will definitely be more beneficial.
Keep in mind the possibility of rearrangements in SN1 reactions which is one reason why the SN1 pathway predominates in 2° and 3° alcohols:
What Makes Tosylates And Mesylates Good Leaving Groups
Lets compare two reactions with a good nucleophile:
How come the first reaction does not work while the second one works very nicely? Why is the tosylate a better leaving group than the OH?
If you recalled the resonance stabilization, well done thats what it is. While the oxygen of the OH group bears the full negative charge on its own, the sulfonate ion has three oxygens to handle the negative charge which is better than the only one in the hydroxyl group:
Recommended Reading: Holt Mcdougal Geometry Workbook Answers
Introducing Tosylates And Mesylates
Is there some way around this? Sure! Its just a matter of replacing that OH group on the sulfur by some kind of relatively inert organic group that doesnt have an acidic proton.
If we swap in a methyl group our leaving group would be OSO2CH3, or methanesulfonate (commonly called, mesylateand abbreviated OMs. This has all the advantages of a great leaving group without the drawback of an acidic proton to react with nucleophiles.
Another popular option is using the conjugate base of p-toluenesulfonic acid, commonly called tosylate and abbreviated OTs.
These groups have essentially identical leaving group ability and for our purposes are interchangeable. Some textbooks tend to use Ts more, others use Ms. It doesnt really matter for us at this stage: they both work.
Conversion Of Alcohols To Alkyl Halides With Socl2 And Pbr3
Thionyl chloride is another great alternative to the HX acids that convert 1° and 2° alcohols to their corresponding alkyl chlorides with inverted chirality. Like for the sulfonyl chlorides, the first step is converting the OH into a good leaving group after which the chloride attacks via an SN2 reaction:
The pyridine is added to ensure the inversion of the stereogenic center via the SN2 mechanism. If pyridine is not present, the reaction tends to go via SNi mechanism.
In some literature, it is mentioned that SOCl2 can convert 3° alcohols as well. Ask your instructor if that would be acceptable.
In a similar reaction, phosphorus tribromide, PBr3, is used to convert 1° and 2° alcohols to their corresponding alkyl bromides with inverted chirality:
Practice
Read Also: Algebra With Pizzazz Answer Key Page 34 Books Never Written
The Mechanism Of Mesylation And Tosylation
Lets discuss the mechanism for converting -2-Butanol to a tosylate followed by a substitution reaction via the SN2 mechanism.
In the first step, the alcohol acts as a nucleophile attacking the sulfur to replace the chloride.
Notice that the carbon with the stereogenic center is not involved in this step and its configuration is still retained:
The pyridine is added as a base to deprotonate the intermediate and speed up the process of forming the Toluenesulfonate ester .
After this step, the OH is now turned into a good leaving group which can be kicked out by a nucleophile:
Mesylation goes by a slightly different mechanism. The first step here is the deprotonation of the acidic proton by the base from methanesulfonyl chloride which forms a sulfene. The sulfene is very electrophilic and reacts quickly with the alcohol:
These reactions will often be shown with the mechanism we saw for the tosylation in undergraduate courses.
Ask your instructor if the same mechanism as for tosylation is acceptable for mesylation.
The formation of the reactive sulfene intermediate gives a slight advantage to mesylate when working with tertiary alcohols since they react very slowly with TsCl.
The advantage of tosylation is that it is a larger molecule and turns some liquid alcohol into solids which sometimes are preferred since they are easier to handle. Also, the aromatic ring of the tosylates allows for better visualization on a TLC plate.