Reactions And Temperature Changes
Energy is conserved in chemical reactions, so the total amount of energy in the universe at the end of a reaction is the same as it was before the reaction.
When a chemical reaction happens, energy is transferred to or from the surroundings. When energy is transferred to the surroundings, this is called an exothermic reaction, and the temperature of the surroundings increases. Examples of exothermic reactions include:
Everyday uses of exothermic reactions include self-heating cans and hand warmers.
When energy is taken in from the surroundings, this is called an endothermic reaction and the temperature of the surroundings decreases. Examples of endothermic reactions include:
- reactions
- the reaction of citric acid and sodium hydrogencarbonate
Everyday uses of endothermic reactions include instant ice packs which can be used to treat sports injuries.
The slideshow describes an exothermic reaction between dilute sodium hydroxide and hydrochloric acid and an endothermic reaction between sodium carbonate and ethanoic acid.
1. Sodium hydroxide solution is poured into a beaker of hydrochloric acid which contains a thermometer showing room temperature
2. The beaker now contains sodium chloride and water, and the thermometer is showing a rise in temperature, so the neutralisation reaction is exothermic
3. Sodium carbonate powder is tipped into a beaker of ethanoic acid which contains a thermometer showing room temperature
Bond Making And Breaking
In all chemical reactions, energy is taken in to break chemical bonds and energy is released when new chemical bonds are made.
The overall energy change of a reaction depends on whether more energy is taken in or is released. You may also find, in some textbooks, H referred to as the enthalpy change.
In an endothermic reaction, more energy is taken in than is released. The energy change in an endothermic reaction is positive.
In an exothermic reaction, more energy is released than is taken in. The energy change in an endothermic reaction is negative.
Bond energies are measured in kJ/mol .
What Chemicals Do Heat And Eat Packets Contain
The Chemicals in Heat Packs
- Calcium chloride is a salt of calcium. Dissolving calcium chloride, commonly known as rock salt, in water is one of the most basic chemical hot packs available.
- Magnesium Sulfate is a sulfate of magnesium. Another chemical that releases a lot of heat when dissolved in water is magnesium sulfate.
- Acetate of sodium.
You May Like: What Is Abiotic In Biology
Exothermic Vs Endothermic Reactions
- The changes in heat content can be determined and measured with a thermometer
- Note that the overall amount of energy does not change as energy is conserved in reactions
- This means that it cannot be or destroyed but it can be transferred
- So, if energy is transferred to the surroundings during a chemical reaction, then the products formed must have less energy than the reactants by the same amount as that transferred
- The following are some examples of heat changes in reactions
- Neutralisation reactions:
- These always give energy out
State The Advantages And Disadvantages Of Fuel Cell
ADVANTAGES
- No harmful gases or waste product is produced
- Waste product is only water so no problem to the environment or disposing off the waste product.
- Do not needs recharging
- Hydrogen is a flammable Gas
- Production of hydrogen depends on non renewable resources.
- Hydrogen being a gas is difficult to store and transport
- Storing and transport of hydrogen involves energy which comes from fossils fuel thereby it contribute indirectly to global warming.
Recommended Reading: Unit 1 Test Study Guide Geometry Basics Answer Key
Heat Exchange In Reactions
- Chemical reactions occur so that elements can achieve a more stable energy state by gaining a full outer shell of electrons
- This is done by chemical bonding where old bonds are broken, and new bonds are formed
- This process involves the transfer of energy into and out of reaction mixtures
- The terms used to describe this are the system and the surroundings
- The energy comes from the chemical bonds themselves which could be considered as tiny stores of chemical energy
- In the majority of reactions, the energy is in the form of heat energy, although sometimes other types of energy are produced such as light or sound
Exam Tip
Physical processes can also involve heat exchange. Examples include freezing or melting which involve a change in state.
Aqa Chemistry Paper 1 Revision: Energy Changes
Beyond Science returns with our final AQA Chemistry Paper 1 blog, exploring energy changes revision. Here, well demystify each aspect of energy changes, so you can confidently tackle this aspect of your exams. Deep breathsoff we go.
You can also subscribe to Beyond Secondary Resources for access to thousands of worksheets and revision tools. Our site was created with teachers in mind and includes lots of teacher instructions, however, it also contains content for students that will be particularly useful when revising! You can and take a look around at our free resources before you subscribe too.
You May Like: Is Paris Jackson Blood Related To Michael
What Chemicals Are Used In Heat And Eat Packs
Chemicals Used in Heat Packs
- Calcium Chloride. One of the simplest chemical hot packs possible involves dissolving calcium chloride, also known as rock salt, into water.
- Magnesium Sulfate. Magnesium sulfate is another chemical that liberates large amounts of heat when dissolved in water.
- Sodium Acetate.
Selfheatingheatselfheatheat
Brady Mergenschroer
Selfheating effectSelfheating
Sophio Schiffelholz
Presentation On Theme: Using Energy Transfers Lo: Describe A Use For The Energy Transferred In A Reaction Explain How The Energy Changes In A Reaction Can Be Used Make Presentation Transcript:
1 Using Energy Transfers LO: Describe a use for the energy transferred in a reaction Explain how the energy changes in a reaction can be used Make a conclusion based on you knowledge of reversible reactions Evaluate the usefulness of using energy processes from chemical reactions Starter With whiteboards… What is the symbol for a reversible reaction? What does exothermic mean? What happens to temperature in an endothermic reaction?
2 Hydrated copper sulphate copper sulphate + water ENDO EXO N 2 + 3 H 2 2 NH 3 EXO ENDO
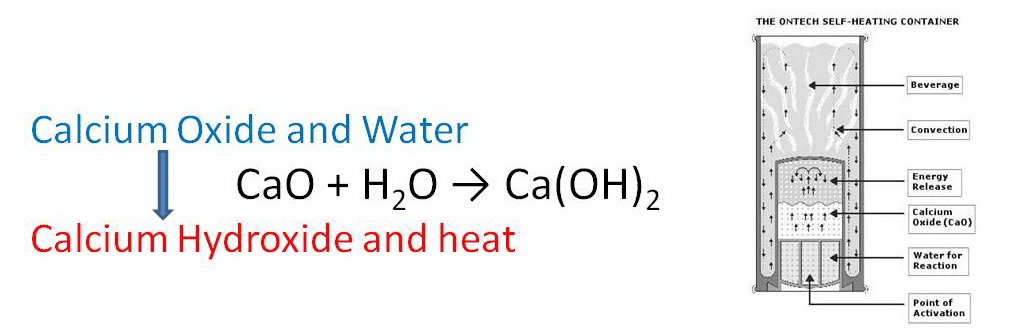
3 Self heating can How does this work ? Think about how this could be self heating. Think about what was done to make it heat up. What was going on ? Hint this might have something to do with previous work.
4 Calcium Oxide Mixes with water Exothermic reaction
5 Around the Room In Groups. Around the room is some information about the uses of exo and endothermic reactions. One person finds the information and reads it. Remember what you can. Report back to the group. Tell them what the information is. If you forget go back and remember a bit more. Someone else in the group finds the next one. Put the information in order.
6 Hand warmers Hand warmers are using the recrystallisation of a solid to produce an exothermic release of energy. How can we reverse this to make it so that the hand warmer can be used again ? Sodium ethanoate solution Sodium ethanoate solid EXO
7 Create a mark scheme
Don’t Miss: Difference Between Human Geography And Physical Geography
How Does Self Heating Food Packaging Work
Self heating food packaging is a packaging technology that uses the heat of the food within the packaging to warm the surrounding air, to keep the food at a steady, desired temperature. The foods temperature can be monitored and adjusted by the user, based on their preference. This method is generally used for keeping food at a constant temperature during transit.
The main process of this technology is to develop and use an electronic heating element that can be embedded in a food rations package through a coating process. This is a process that applies to almost all the food package. Foods that are heated through this process includes pies/cakes, bread, oatmeal, and some other foods.
In the quest to achieve the perfect meal, we often overlook how its been prepared and how the packaging affects our food preparation. Before you eat a meal, you should read and think about how the food was prepared. Splendid design of food packaging makes a huge difference.
Self-heating food packaging is active packaging that can heat food without the need of external heat sources or electricity. Exothermic chemical reactions are often used in packets. Self-cooling packets are another option.
How does a self-heating meal function in this regard?
Aside from that, GCSE, how do self-heating cans work?
Why are self-heating cans only good for one use?
Answers to Related Questions
Which Chemical Process Generates The Greatest Amount Of Heat
exothermic reaction
Self heating food packaging is a kind of packaging material or a food container that contains a thermal energy generator. It can keep the food temperature at the set temperature even after the product is opened, turning off the heating part of the system directly. The food can be kept warm for a long time without increasing its temperature.. Read more about self-heating food packaging malaysia and let us know what you think.
Recommended Reading: Houghton Mifflin Geometry Workbook Answers
Exothermic And Endothermic Reactions
When a chemical reaction takes place, energy is involved. Energy is transferred when chemical bonds are broken and when new bonds are made.
Exothermic reactions involve the transfer of energy from the reacting chemicals to the surroundings. During a practical investigation, an exothermic reaction would show an increase in temperature as the reaction takes place.
Examples of exothermic reactions include:
- Combustion
- Respiration
- Neutralisation
We can use exothermic reactions in a number of ways, examples of ways we use exothermic reactions include:
- Hand warmers
- Self heating cans used in camping
Endothermic reactions involve the transfer of energy from the surroundings to the reacting chemicals. During a practical investigation, an endothermic reaction would show a as the reaction takes place.
Examples of endothermic reactions are rarer than exothermic but include:
What Are The Two Main Types Of Thermodynamic Reactions

Exothermic reactions are reactions that release energy in the form of heat. You are probably familiar with many examples of these reactions. For example, burning gasoline in a cars engine is an exothermic reaction. This particular type of exothermic reaction is known as a combustion reaction. A combustion reaction occurs when a compound, such as the hydrocarbons that make up fuel, react with oxygen to form a new product and produce heat.
Endothermic reactions are the opposite of exothermic reactions. They absorb heat energy from their surroundings. This means that the surroundings of endothermic reactions are colder as a result of the reaction. Melting ice is an example of this type of reaction.
Read Also: Unit 1 Geometry Basics Segment Addition Postulate
What Is The Maximum Temperature That Mre Heaters Can Reach
This is exactly what our soldiers use in the field! A simple mixture of powdered food-grade iron and magnesium, salt, and water is used to make the heater. When water is put over the accompanying heating pad, it produces enough heat to reheat the pre-cooked food to a temperature of 100 degrees Fahrenheit in about 12 minutes.
Heating Any Of Our Self
Spent heaters are biodegradable and easily disposed of as ordinary household trash. There are no toxic materials.
Cafe2Go Self-Heating Beverage Kits heat much the same way.
The patented TRUETECH Self-Heating Technology was introduced for use as the Field Ration Heater by the U.S. Armed Forces in 1990 to heat Meals, Ready-to-Eat for soldiers in the field. Weve heated nearly 2 Billion MREs since then.
Our self-heating technology has been written about on numerous occasions. A more in-depth explanation can be found in this excerpt from ChemMaters Magazine.
Don’t Miss: Age Word Problems With 3 Variables
Calculations Using Bond Energies
Bond energies are used to calculate the change in energy of a chemical reaction.
If we try to calculate the change in energy for the reaction:
2H202 2H2O + O2
Reactions will often be shown as displayed formulae so it is easier to work out which bonds are being broken and which bonds are being made.
H-O-O-H H-O-H
On the left-hand side of the equation, the bonds are breaking.
There are four O-H bonds and two O-O bonds.
So, + = 2148
On the right-hand side there are four 0-H bonds and one O=O bond being made.
So, + 498 = 2354
H = sum sum
H = 2148 2354 = -206 kJ/mol
The reaction is exothermic as H is negative.
C25 Exothermic And Endothermic Reactions
When chemical reactions occur, energy is transferred to or from the surroundings.
Importantly, it is the particular process of the chemical reaction, that either generates heat to, or absorbs heat from, the surroundings. A common misconception is that students believe that the reactants alone release heat or absorb heat-this is incorrect!.
Exothermic reaction
An exothermic chemical reaction is a reaction that results in the generation of heat energy as well as the chemical products of the reaction.In doing so, REACTANTS gives rise to both HEAT ENERGY and PRODUCTS in an exothermic chemical reaction.
A classic example of this is the combustion of methane gas by a Bunsen burner
Methane + Oxygen Carbon Dioxide + Water + Heat energyCH4 + 2O2 CO2 + 2H2O + Heat energy
The heat energy that is produced, as a consequence of the chemical reaction, is transferred to the surroundings, hence the air surrounding a Bunsen flame heats up.
Apart from combustion, other examples of exothermic reactions include: many oxidation and acid/base neutralisations. Everyday uses of exothermic reactions include self-heating cans and hand warmers.
An exothermic energy level diagram
Because chemical stored energy is released as heat energy during the chemical reaction, it follows that:
energy stored by the chemical bonds in the reactants > energy stored by the chemical bonds of the products
Hence, the reactants are situated at a higher chemical potential energy level than the products.
You May Like: Definition Of Abiotic In Science
Is Neutralization An Exothermic Or An Endothermic Process
An acid and a base combine to produce salt and water in a neutralization reaction. Its also crucial to realize that during an reaction that is exothermic, bonds are formed and energy is released into the environment. This is what occurs towards the end of a neutralization process, giving it an exothermic nature.
Endothermic And Exothermic Reactions 2
Exothermic reactions release energy into the surroundings, whereas endothermic reactions absorb energy. Hand warmers, glow sticks and self heating coffee are all applications of energy changes in chemical reactions. For the higher tier of GCSE Chemistry, you will be expected to explain how and why these energy changes occur. The short answer to ‘how‘ is that during a chemical reaction, bonds are broken and made. The short answer to ‘why‘ is that energy is involved in making and breaking bonds.
In a chemical reaction, you always end up with the same numbers and types of atom as you started with, but joined together in a different combination. For example, the reaction between hydrogen and oxygen to give water. To start with, the hydrogen atoms are joined to each other in pairs, as are the oxygen atoms. At the end of the reaction, two hydrogen atoms are joined to one oxygen atom in the compound water. Clearly, to get to this state of affairs, the bond between the hydrogen atoms must have been broken and so must the bonds between the oxygen atoms. Only when that has occured can the bonds of the water molecule form.
You May Like: Half-life Formula Chemistry
How Do Self
Self-heating cans have dual chambers, one surrounding the other. The chemicals are in the inner chamber and the beverage surrounds it in the outer chamber. When the user wants to heat the contents of the can, they push on the bottom of the can to break the barrier separating the water from the chemicals.
How Do Re

1. A hand warmer contains sodium acetate, dissolved in water. The solution is super-saturated, which means it has been heated to dissolve more sodium acetate. The solution crystallises readily.
2. When the internal metal strip is bent, tiny bits of metal are released, which offer nucleation sites for crystals to form.
3. As the crystals spread, the stored heat energy of the solution is released, heating the hand warmer up to 54°C an exothermic reaction.
4. The hand warmer can be reset by boiling it in a pan of water to liquefy the crystals.
to BBC Focus magazine for fascinating new Q& As every month and follow on Twitter for your daily dose of fun science facts.
- Try 3 issues for just £5
- Receive every issue delivered direct to your door with FREE UK delivery
Also Check: Holt Mcdougal Larson Geometry Workbook Answers
How Self
The patented TRUETECH Self-Heating Technology is a simple combination of food grade iron and magnesium powder, salt, and water. When the contents of the water pouch are poured over the heater pad, the Food Heater releases enough heat to warm-up a pre-cooked meal 100 degrees Fahrenheit in approximately 10 minutes.