An Easy Explanation Of How To Find Percent Abundance
According to chemistry principles, isotopes have same atomic number but different mass number. Abundance is defined as the amount of isotope contained in its parent element. This ScienceStruck post tells you how to calculate percent abundance for any element having isotopes.
Like it? Share it!
According to chemistry principles, isotopes have same atomic number but different mass number. Abundance is defined as the amount of isotope contained in its parent element. This ScienceStruck post tells you how to calculate percent abundance for any element having isotopes.
NoteAn element can consist of number of isotopes, and it is necessary to consider all of its isotopes while computing the percent abundance. Consider element carbon , it has 15 isotopes, and it is mandatory to consider all of them.
Every element has atoms which consist of protons, electrons, and neutrons. When an element has more than one form having same number of protons but different number of neutrons in the nucleus, they are called isotopes. They either occur naturally or are artificially produced. The element chlorine has more than 50 isotopes, of which Cl-35 and Cl-37 are two stable isotopes. They have same atomic number, which is 17 however, their mass number is 35 and 37, respectively.
Definition of percentage abundance for X in Y can be best explained in two possible ways as follows:
1. how many percent of Y is X.2. how much of X is in Y
Average mass of an element= +
The Core Difference Lies In The Nucleus: Neutrons
To get to the core of why isotopes have varying mass but similar chemical properties, we have to examine their atomic structure.
Isotopes have the same number of protons but different number of neutrons packed into the nucleus. Since the neutron has a relative mass of 1, having more neutrons will increase the relative mass of an isotope. For example, hydrogen-3 is the heaviest isotope of hydrogen as it has the most number of neutrons.
But neutrons are electrically neutral. Having more of them does not change the overall positive charge of the nucleus, which will attract the same number of electrons. With the same electronic configuration, isotopes form the same type of bond and have similar chemical properties. This is evident in the isotopes of hydrogen, as they all have a single electron each.
Isotopes are defined as atoms of the same element with the same number of protons but different number of neutrons. Consequently, they have the same atomic number but different mass number.
Isotopes Are Atoms Of The Same Element With Different Masses
Atoms of the same element can have different mass. We say that they are isotopes of the same element.
For example, hydrogen has three isotopes with different relative mass, which is indicated by the number behind their name. So hydrogen-1 has a relative mass of 1, while hydrogen-2 has twice the mass. Hydrogen-3 is the heaviest isotope, with a relative mass of 3.
While their relative mass is different, isotopes are like peas in a pod in terms of chemical properties. They react in an almost identical way, making it impossible to distinguish them through reactions. For example, all three isotopes of hydrogen react explosively with oxygen in a 2:1 ratio.
Isotopes of the same element have different physical properties but similar chemical properties.
Dont Miss: Exponential Growth And Decay Worksheet Answer Key Algebra 1
Recommended Reading: Bridge To Algebra Answers
What Is Relative Abundance Of Isotopes
The relative abundance of isotopes of an element is determined by mass spectrometry. The properties of an element mostly correspond to the most abundant isotopes of that element.
More than 280 isotopes are known, which occur in nature, out of which 40 are radioactive.
Moreover, about 300 artificial isotopes have been produced through disintegration.
Use The Relative Abundance Concept
Then, we will use the relative abundance formula for the given problem. As the given element consists only two isotopes, an equation can be set by the given equation below
M1x+M21-x=MA
Where,
M1 is the mass of one isotope, x is the relative abundance, M2 is the mass of other isotope and MA is the average atomic mass of the element
As we have, the atomic mass of chlorine-35 is 34.97 and the atomic mass of chlorine-37 is 36.97 amu. So, now lets find the relative abundance.
Here, we need to solve for unknown x which is the relative abundance. One isotope is related as M1 and the other as M2.
We have
Placing the data in the first equation, we get
34.97×x+36.97×1-x=35.45
Also Check: Holt Geometry Chapter 7 Test Form B Answers
What Is The Unit For Relative Abundance
Note that relative abundance has no units . Alternatively, relative abundances can be expressed as a percentage. Species composition A list of all the species in this defined unit, along with some measure of the abundance .
Use the following formula for relative abundance chemistry problems:
What If Elements Have More Than Two Isotopes
For example:
Oxygen has three naturally occurring isotopes 16O, 17O and 18O. The average atomic mass of oxygen is 15.9994 amu. Given, the atomic weight of 16O is 15.995 amu, 17O is 16.999 amu and 18O is 17.999 amu. Also, 17O has 0.037 percent in nature. What are the other isotopes percent abundances?
Firstly, we have the abundance of one isotope which is 0.0037. So, the abundances of the other two remaining isotopes is = 0.99963.
Let x be the unknown abundance of 16O and other isotope abundance of 18O be .
Now, modifying equation , we get
* + * + * = 15.9994
or 15.995x 17.999x = 15.9994
or x = 0.9976
So, we get the abundance of 16O is 0.9976 and the abundance of 18O is = 0.00203.
Hence, the percent abundance of three isotopes are given by
16O=0.9976*100=99.76%
Also Check: Paris Jackson Biological Parents
What Does Relative Abundance Represent
Relative species abundance is a component of biodiversity and refers to how common or rare a species is relative to other species in a defined location or community. Relative abundance is the percent composition of an organism of a particular kind relative to the total number of organisms in the area.
What Is The Natural Abundance Of Potassium
The three naturally occurring isotopes of potassium are $\ce$ $\ce$ and $\ce$.
The percent natural abundances of $\ce$ and $\ce$ are $93.2581\%$ and $6.7302\%$ respectively.
a) What is the natural abundance of $\ce$?
b) Determine the isotopic mass of $\ce$.
So I started to utilize this equation:
$\mathrm$
But then I realized that I did not have some values. $\ce$ is the only isotope which has two values. $\ce$ only has the mass weight, however, if I were to multiply $\ce$s mass with would that help me arrive at an answer?
Also, would that answer be accurate since it only takes into account that there are only to values that add up to 100%.
Recommended Reading: Holt Mcdougal Geometry Practice Workbook
Does Relative Abundance Have To Equal 100
It is actually a weighted mass of the elements isotopes and their relative abundance. You know that the sum of the percentages of the isotopes is equal to 1 , so the relative abundance of the isotopes can be found using simple algebra.
Use the following formula for relative abundance chemistry problems:
Relative Abundance Of Mixture In Mass Spectra
In MS, as far as I know, the $y$-axis, relative abundance means the number of ions detected. Then does ratio of heights of two peaks stand for molar ratio, not mass ratio
For example, given that I analyze a mixture of two components A and B using MS, and there appears only molecular ion peak , then is the ratio of heights of two peaks equal to molar ratio of A and B?
- 3$\begingroup$No. There are all sorts of ionisation effects that influence the peak heights that have nothing to do with the molar ratios. Phosphine oxides for example ionise very well and a small amount can completely dominate a MS spectrum.$\endgroup$
There are universal detectors with which you can compare signals of different substances. But MS detectors are not universal meaning that high intensity of the signal doesn’t necessarily mean high concentration. And vice versa.
Whether the signal is high or not will depend on how well the compound is ionized. So in case of you A& B compounds:
- A may be well-ionized and thus the signal will be high even though the concentration is low
- B is poorly ionized and you may not even see a peak even though the concentration may be high.
To identify how much of analyte you have you first need to run A with a known concentration so that you determine the relationship between area and concentration. Then if you use the same LCMS conditions you can run A with your to-be-determined concentration and by ratios of peak areas you can determine the unknown concentration.
You May Like: Glencoe Algebra 1 Chapter 4 Test Form 2b Answer Key
What Is Relative Abundance
Relative abundance of an element is a measure of the occurrence of an element relative to all other elements in the environment. There are three ways to determine the relative abundance of an element:
Volume fraction method is most common for gaseous elements in gas mixtures, i.e, the atmosphere of earth. However, most relative abundance expressions are mass fractions.
Figure 2: A Graph Showing Relative Abundance of Elements on Earths Upper Crust
When considering the universe, the most abundant chemical elements are hydrogen and helium. When considering the earth, the most common element is iron whose mass percentage is 32.1%. Other elements are oxygen , silicone , magnesium , sulfur and other elements are present in trace percentages.
Summary Percent Abundance Vs Relative Abundance
![Relative atomic mass_& _mass_spectrometry[1][1]](https://www.tutordale.com/wp-content/uploads/relative-atomic-mass_-_mass_spectrometry11.jpeg)
Percent abundance and relative abundance are two terms used to give the abundance of isotopes and chemical elements. The key difference between percent abundance and relative abundance is that percent abundance gives the abundance of isotopes whereas relative abundance gives the abundance of chemical elements.
Reference:
1. Average Atomic Mass. Average Atomic Mass, , Available here.2. Abundance of the chemical elements. Wikipedia, Wikimedia Foundation, 25 Feb. 2018, Available here.3. Simoes, Christian. Abundance of the chemical elements. Astrono, Available here.
Image Courtesy:
1. Isotope CNO By Lucquessoy Own work via Commons Wikimedia2. Elemental abundances By Gordon B. Haxel, Sara Boore, and Susan Mayfield from USGS vectorized by User:michbich via Commons Wikimedia
Also Check: Figure Definition Psychology
How Does Isotope Abundance Impact Atomic Weight
Atomic mass depends on the composition of protons and neutrons in an element, with each weighing 1 atomic mass unit . Electrons are an important part of elements as well, but they have such a small mass that they are considered negligible when calculating atomic mass. As both protons and neutrons make up an atoms mass, when an element differs in its number of neutrons, it is impactful on the mass.
Though they sound like synonyms, atomic mass and atomic weight are different. Isotopes impact the value of both. Atomic mass is defined as the mass of an individual atom of an element. This is solely the calculation of the weight of protons and neutrons in amu. Atomic weight on the other hand is the weighted average of all of the isotopes of an element that exist. This is where isotope abundance comes in. Though there may be many naturally occurring isotopes of an element, they do not exist in equal amounts. There are many isotopes that occur much more commonly than others, and therefore have a greater impact on the atomic weight. If given the atomic mass of the isotopes of an element as well as their relative abundances, we can follow simple steps to calculate the atomic weight.
Isotopic Abundance And Atomic Weight
- Calculate atomic weight from percent abundance
- Manipulate the atomic weight equation to calculate various unknown variables
- Distinguish between atomic weight, atomic number, and mass number
Introduction
As mentioned in the previous section, atoms that have the same atomic number , but different mass numbers are called isotopes. There are naturally occurring isotopes and isotopes that are artificially produced. Of all the elements on the periodic table, only 21 are pure elements. Pure, or monotopic, elements are those elements with only one naturally occurring isotope. The following lists the 21 pure elements:
| \ |
Table of the 2.3.1 Monotopic Elements
You May Like: What Are The Different Branches Of Chemistry
Using Isotope Abundance To Calculate Atomic Weight
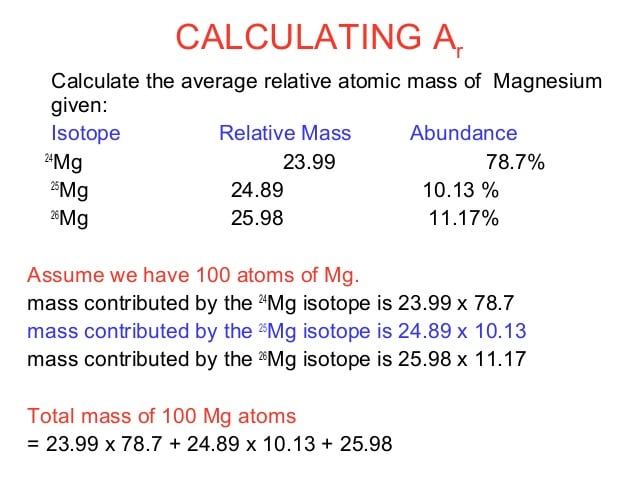
As stated previously, the number of isotopes and their percent abundance are all that are needed to calculate the atomic weight of an element. We can start by using magnesium as an example. Magnesium has three naturally occurring isotopes: 24Mg, 25Mg, and 26Mg. Each isotope has an abundance of 78.70 %, 10.13%, and 11.17%, respectively. The atomic mass of each isotope is usually very close to each isotope value. In this example, the mass of each isotope is 23.985 amu, 24.985 amu, and 25.982 amu respectively.
Now that we have all of the information about mass and abundance, we can calculate the atomic weight of magnesium. If you have trouble visualizing all of the values, you can organize them in a table to make your information more clear.
| Isotope | |
| 25.982 | 11.17% |
We start by multiplying each isotopes mass by its abundance. This can be done in two ways. First, we can directly multiply the mass by the percent:
23.985 amu = 1887.6 amu
On the other hand, we can change the percent to a decimal out of one and then multiply by the mass. This can be done by dividing the percent by 100.
23.985 amu = 18.876 amu
With both of these methods, the next step is to repeat for the other isotopes and add the values together.
Method 1:
23.985 amu + 24.985 amu + 25.982 amu =
1887.6 amu + 253.09 amu + 290.21 amu = 2430.90 amu
Method 2:
23.985 amu + 24.985 amu + 25.982 amu = 24.3090 amu
2430.90 amu/100= 24.3090 amu
Membership Of The Sponsoring Body
Membership of the IUPAC Inorganic Chemistry Division Committee for the period 2014â2015 was as follows:
President: J. Reedijk Secretary: M. Leskelä Vice President: L. R. Ãhrström Past President: R. D. Loss Titular Members: T. Ding M. Drábik E. Y. Tshuva D. Rabinovich T. Walczyk M. E. Wieser Associate Members: J. Buchweishaija J. Garcia-Martinez P. Karen A. Kilic K. Sakai R.-N. Vannier National Representatives: F. Abdul Aziz L. Armelao A. Badshah V. Chandrasekhar J. Galamba Correia S. Kalmykov L. Meesuk S. Mathur B. Prugovecki N. Trendafilova .
Membership of the IUPAC Commission on Isotopic Abundances and Atomic Weights for the period 2014â2015 was as follows:
Chair: J. Meija Secretary: T. Prohaska Titular Members: W. A. Brand M. Gröning R. Schönberg X.-K. Zhu Associate Members: T. Hirata J. Irrgeher J. Vogl National Representatives: P. De Bièvre T. B. Coplen Ex-officio member: J. Reedijk .
Don’t Miss: Formal Charge Of Cf4
Abundance Of The Chemical Elements
The abundance of the chemical elements is a measure of the occurrence of the chemical elements relative to all other elements in a given environment. Abundance is measured in one of three ways: by the mass-fraction by the mole-fraction or by the volume-fraction. Volume-fraction is a common abundance measure in mixed gases such as planetary atmospheres, and is similar in value to molecular mole-fraction for gas mixtures at relatively low densities and pressures, and ideal gas mixtures. Most abundance values in this article are given as mass-fractions.
For example, the abundance of oxygen in pure water can be measured in two ways: the mass fraction is about 89%, because that is the fraction of waters mass which is oxygen. However, the mole-fraction is about 33% because only 1 atom of 3 in water, H2O, is oxygen. As another example, looking at the mass-fraction abundance of hydrogen and helium in both the Universe as a whole and in the atmospheres of gas-giant planets such as Jupiter, it is 74% for hydrogen and 2325% for helium while the mole-fraction for hydrogen is 92%, and for helium is 8%, in these environments. Changing the given environment to Jupiters outer atmosphere, where hydrogen is diatomic while helium is not, changes the molecular mole-fraction , as well as the fraction of atmosphere by volume, of hydrogen to about 86%, and of helium to 13%.
Have You Ever Tried To Move A Boulder
You have a pile of rocks to move and need to decide what equipment you want to rent to move them. If the rocks are fairly small, you can get a shovel to pick them up. Larger rocks could be moved by hand, but big boulders will need some sort of mechanical scoop. The amount of each kind of rock will also determine how much time you will need to get the job done. Knowing the relative amounts of large, medium, and small rocks can be very useful in deciding how to approach the job.
Most elements occur naturally as a mixture of two or more isotopes. Table below shows the natural isotopes of several elements, along with the percent natural abundance of each.
| Element | ||
| ^_\text | 30.83 | 64.928 |
For some elements, one particular isotope predominates greatly over the other isotopes. Naturally occurring hydrogen is nearly all hydrogen-1 and naturally occurring oxygen is nearly all oxygen-16. For many other elements, however, more than one isotope may exist in more substantial quantities. Chlorine is a yellowish-green toxic gas. About three quarters of all chlorine atoms have 18 neutrons, giving those atoms a mass number of 35. About one quarter of all chlorine atoms have 20 neutrons, giving those atoms a mass number of 37. Were you to simply calculate the arithmetic average of the precise atomic masses, you would get 36.
\displaystyle\frac=35.968\text
Sample Problem: Calculating Atomic Mass
Known
Unknown
Step 2: Calculate
Recommended Reading: Holt Mcdougal Geometry Workbook Answers