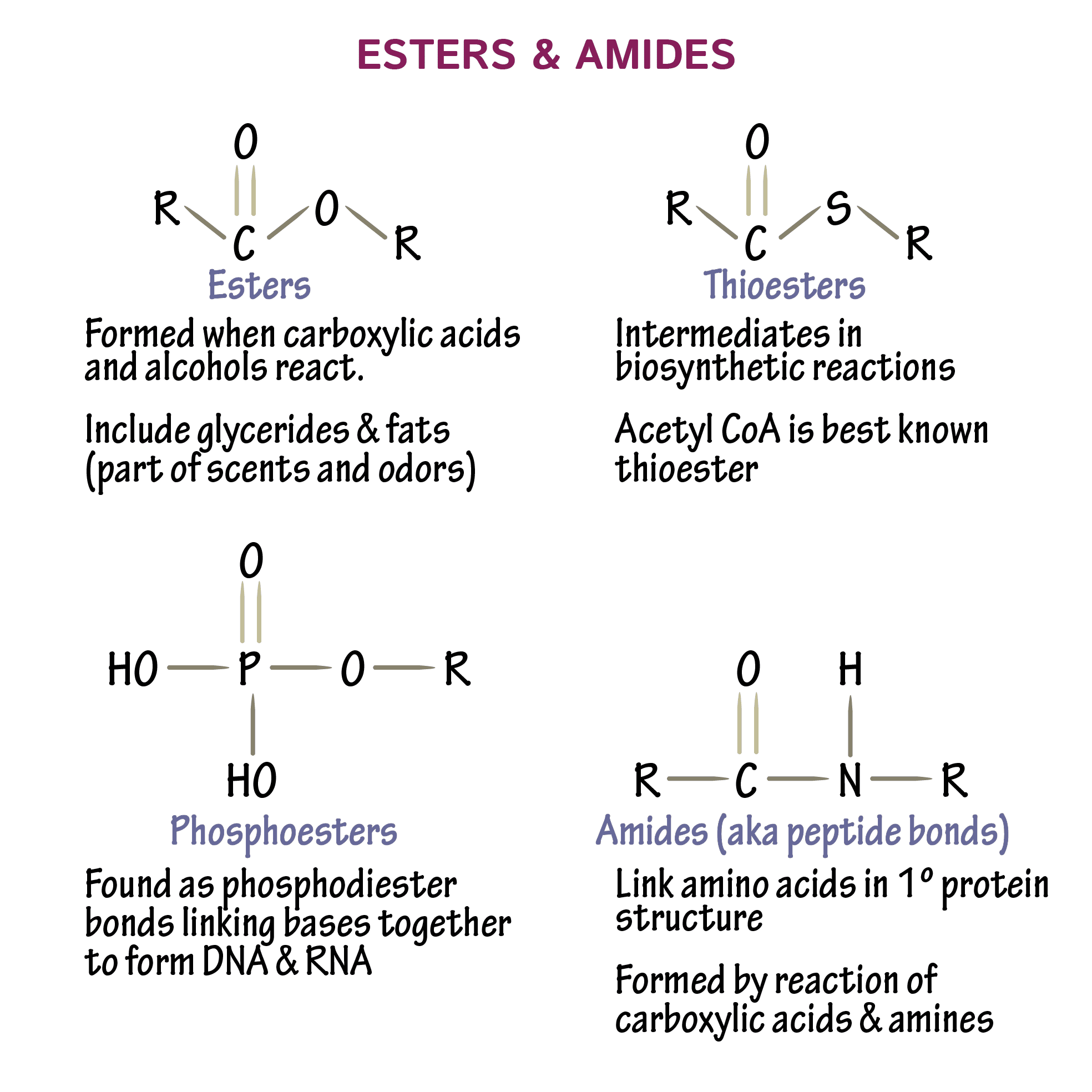
What Makes An Ester
Esters are usually derived from an inorganic acid or organic acid in which at least one hydroxyl group is replaced by an alkoxy group, and most commonly from carboxylic acids and alcohols. Esters occur widely in nature many naturally occurring fats and oils are the fatty acid esters of glycerol.
What Is An Ester
When a hydroxyl group is replaced by an alkoxy group in a chemical compound generated from an acid , the result is known as an ester. To put it simply, esters are a class of chemical compounds created by the bonding of an alcohol group with a collection of organic acids while leaving water molecules.
A Closer Look: Condensation Polymers
A commercially important esterification reaction is condensation polymerization, in which a reaction occurs between a dicarboxylic acid and a dihydric alcohol , with the elimination of water. Such a reaction yields an ester that contains a free carboxyl group at one end and a free alcohol group at the other end. Further condensation reactions then occur, producing polyester polymers.
The most important polyester, polyethylene terephthalate , is made from terephthalic acid and ethylene glycol monomers:
Polyester molecules make excellent fibers and are used in many fabrics. A knitted polyester tube, which is biologically inert, can be used in surgery to repair or replace diseased sections of blood vessels. PET is used to make bottles for soda pop and other beverages. It is also formed into films called Mylar. When magnetically coated, Mylar tape is used in audio- and videocassettes. Synthetic arteries can be made from PET, polytetrafluoroethylene, and other polymers.
Recommended Reading: What Is Intrinsic Motivation In Psychology
Ester Bonds In Prodrugs
ACS Chem. Biol.
Publication History
Article Views are the COUNTER-compliant sum of full text article downloads since November 2008 across all institutions and individuals. These metrics are regularly updated to reflect usage leading up to the last few days.
Citations are the number of other articles citing this article, calculated by Crossref and updated daily. Find more information about Crossref citation counts.
The Altmetric Attention Score is a quantitative measure of the attention that a research article has received online. Clicking on the donut icon will load a page at altmetric.com with additional details about the score and the social media presence for the given article. Find more information on the Altmetric Attention Score and how the score is calculated.
A recent study challenges the oft-held notion that ester bonds in prodrug molecules are cleaved rapidly and completely inside cells by endogenous, nonspecific esterases. Structureactivity relationship studies on acylated sugars reveal that regioisomeric compounds display disparate biological activity, suggesting that ester bonds can persist in a cellular context.
Alkylation Of Carboxylate Salts
Although not widely employed for esterifications, salts of carboxylate anions can be alkylating agent with alkyl halides to give esters. In the case that an alkyl chloride is used, an iodide salt can catalyze the reaction . The carboxylate salt is often generated in situ. In difficult cases, the silver carboxylate may be used, since the silver ion coordinates to the halide aiding its departure and improving the reaction rate. This reaction can suffer from anion availability problems and, therefore, can benefit from the addition of phase transfer catalysts or highly polar such as DMF.
Recommended Reading: What Are Physical Features In Geography
Claisen Condensation And Related Reactions
As for aldehydes, the hydrogen atoms on the carbon adjacent the carboxyl group in esters are sufficiently acidic to undergo deprotonation, which in turn leads to a variety of useful reactions. Deprotonation requires relatively strong bases, such as alkoxides. Deprotonation gives a nucleophilic enolate, which can further react, e.g., the Claisen condensation and its intramolecular equivalent, the Dieckmann condensation. This conversion is exploited in the malonic ester synthesis, wherein the diester of malonic acid reacts with an electrophile , and is subsequently decarboxylated. Another variation is the FráterSeebach alkylation.
What Is An Ester In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
An ester is an organic compound where the hydrogen in the compound’s carboxyl group is replaced with a hydrocarbon group. Esters are derived from carboxylic acids and alcohol. While carboxylic acid has the -COOH group, the hydrogen is replaced by a hydrocarbon in an ester. The chemical formula of an ester takes the form RCO2R, where R is the hydrocarbon parts of the carboxylic acid, and R is the alcohol.
The term “ester” was coined by the German chemist Leopold Gmelin in 1848. It is likely the term was a contraction of the German word “essigäther,” which means “acetic ether.”
Recommended Reading: What Does Grid Mean In Geography
Reduction Of Acid Chlorides
Esters or carboxylic acids cannot be reduced to aldehydes by hydride reducing agents because aldehydes are more reactive than esters or carboxylic acids. Acid chlorides are more reactive than esters toward nucleophilic hydride ion. As a result, acid chlorides are more rapidly reduced than esters. However, lithium aluminum hydride is such a strong reducing agent that acid chlorides are still reduced all the way to primary alcohols. The milder reducing agent lithium aluminum tri hydride reacts with acid chlorides but much more slowly with aldehydes, which can therefore be isolated.
Matilde V. Solmi, … Walter Leitner, in, 2019
Carboxylic Acids And Esters
The odor of vinegar is caused by the presence of acetic acid, a carboxylic acid, in the vinegar. The odor of ripe bananas and many other fruits is due to the presence of esters, compounds that can be prepared by the reaction of a carboxylic acid with an alcohol. Because esters do not have hydrogen bonds between molecules, they have lower vapor pressures than the alcohols and carboxylic acids from which they are derived .
Figure 2. Esters are responsible for the odors associated with various plants and their fruits.
Both carboxylic acids and esters contain a carbonyl group with a second oxygen atom bonded to the carbon atom in the carbonyl group by a single bond. In a carboxylic acid, the second oxygen atom also bonds to a hydrogen atom. In an ester, the second oxygen atom bonds to another carbon atom. The names for carboxylic acids and esters include prefixes that denote the lengths of the carbon chains in the molecules and are derived following nomenclature rules similar to those for inorganic acids and salts :
The functional groups for an acid and for an ester are shown in red in these formulas.
The hydrogen atom in the functional group of a carboxylic acid will react with a base to form an ionic salt:
Carboxylic acids are weak acids , meaning they are not 100% ionized in water. Generally only about 1% of the molecules of a carboxylic acid dissolved in water are ionized at any given time. The remaining molecules are undissociated in solution.
Recommended Reading: Geometry Common Core Book Answers
Physical Properties And Characterization
Esters are more polar than ethers but less polar than alcohols. They participate in hydrogen bonds as hydrogen-bond acceptors, but cannot act as hydrogen-bond donors, unlike their parent alcohols. This ability to participate in hydrogen bonding confers some water-solubility. Because of their lack of hydrogen-bond-donating ability, esters do not self-associate. Consequently, esters are more volatile than carboxylic acids of similar molecular weight.
Physical Properties Of Ester:
- Esters of lower acids are colorless, pleasant-smelling solids, while those of higher acids are solids.
- Esters are less polar than alcohols and more polar than ethers.
- Methyl and ethyl esters have lower boiling points than their respective parent acids.
- Lower esters are largely water soluble. Esters are rapidly less soluble in water as their mass increases.
- Unlike their parent alcohols, they can participate in hydrogen bonds as hydrogen-bond acceptors but not as donors. This capacity for hydrogen bonding confers some degree of water solubility.
- Esters cannot self-assemble due to their inability to donate hydrogen bonds. Therefore, compared to carboxylic acids of the same molecular weight, esters are more volatile.
Read Also: Algebra 1 Eoc Practice Test
What Is An Ester Functional Group
In organic chemistry, esters are a common functional group. The basic structure of an ester consists of a carbon single bonded to carbon, double bonded to oxygen, and single bonded to oxygen. For the molecule below, the simple structure of an ester group is shown.
The basic chemical formula of an ester is R-COOR. The R groups denote the rest of the molecule not included in the functional group.
What is an Ester Bond?
An ester bond is a linkage between an atom that is double bonded to an oxygen atom bearing any alkyl or aryl group. Ester bonds are found in ester functional groups. In addition, the bond is generally covalent and is essential in the formation of lipids.
These ester bonds are important when it comes to lipids in our bodies. When glycerol combines with fatty acid molecules, it allows for three esters to be produced. This process is what causes ester bonds to form.
How Do Esters Work
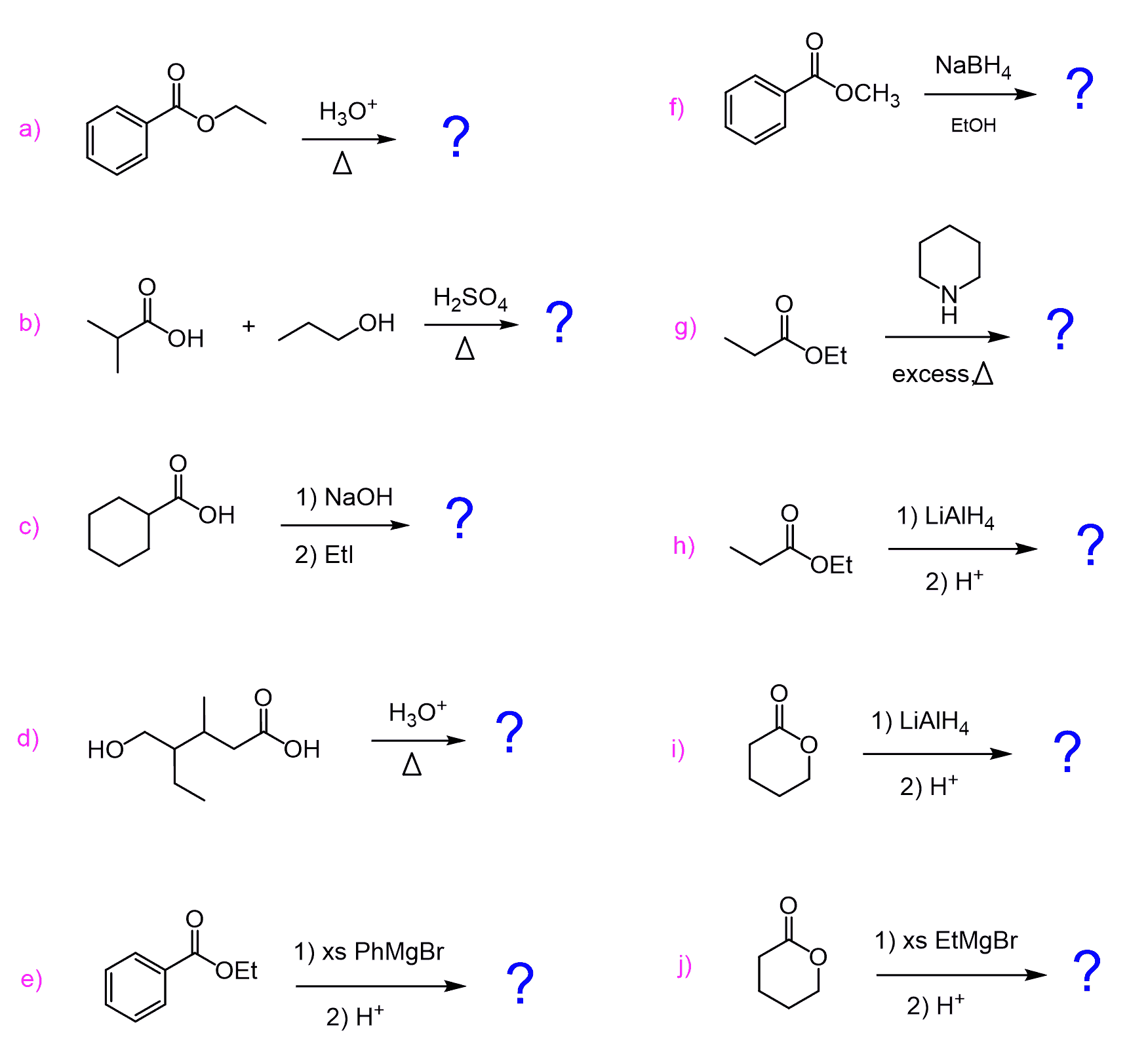
Esters undergo hydrolysis under acidic and basic conditions. Under acidic conditions, the reaction is the reverse reaction of the Fischer esterification. Under basic conditions, hydroxide acts as a nucleophile, while an alkoxide is the leaving group. This reaction, saponification, is the basis of soap making.
Also Check: Houghton Mifflin Harcourt Publishing Company Geometry
Chemical Properties Of Vegetable Oils
Reactions at ester region
Ester functionality is common for all vegetable oils and traditional oleochemicals are produced by modifying this region. More than 90% of oleochemical reactions occur at the ester region . Generally, two reactions such as hydrolysis and transesterification are possible in the ester regions which produce basic chemicals such as fatty acids, glycerol, fatty acid alkyl esters .
Figure 4.8. Chemical transformations of oils including hydrolysis and transesterification.
These basic chemicals can be used as such or it can be further converted into important derivatives by chemical transformations. Intermediate chemical substances produced from these basic oleochemical substances are alcohol ethoxylates/sulfates, alcohol ether sulfates, quaternary ammonium salts, monoacylglycerols , diacylglycerols , structured triacylglycerols and sugar esters.
Reactions at fatty chain region
Oleochemicals are produced through reaction chemistry involving the fatty chain groups. Structural alterations in the fatty chain of the vegetable oils give ample scope for the preparation of new kind of oleochemicals. There are many synthetic protocols for modifying fatty chain regions which result in different products. The important reactions are discussed below and corresponding products mentioned in the brackets.
James G. Speight PhD, DSc, in, 2011
Example : Oxidation And Reduction In Organic Chemistry
Methane represents the completely reduced form of an organic molecule that contains one carbon atom. Sequentially replacing each of the carbon-hydrogen bonds with a carbon-oxygen bond would lead to an alcohol, then an aldehyde, then a carboxylic acid , and, finally, carbon dioxide:
}_\rightarrow}_\text\rightarrow}_\text\rightarrow}_\text\rightarrow}_
What are the oxidation numbers for the carbon atoms in the molecules shown here?
In this example, we can calculate the oxidation number for the carbon atom in each case . For CH4, the carbon atom carries a 4 oxidation number . For the alcohol , the carbon atom has an oxidation number of 2 for the aldehyde, the carbon atoms oxidation number is 0 for the carboxylic acid, the carbon atoms oxidation number is +2 and for carbon dioxide, the carbon atoms oxidation number is +4 .
Check Your Learning
Indicate whether the marked carbon atoms in the three molecules here are oxidized or reduced relative to the marked carbon atom in ethanol:
There is no need to calculate oxidation states in this case instead, just compare the types of atoms bonded to the marked carbon atoms:
Aldehydes are commonly prepared by the oxidation of alcohols whose OH functional group is located on the carbon atom at the end of the chain of carbon atoms in the alcohol:
Alcohols that have their OH groups in the middle of the chain are necessary to synthesize a ketone, which requires the carbonyl group to be bonded to two other carbon atoms:
Recommended Reading: What Is Psychology Foundations Applications And Integration
Why Do Esters Smell
Esters smell sweet because of the feeble intermolecular forces they show. This encourages ester molecules to penetrate and hit the nose in the gas phase. To share in hydrogen bonding, there are not highly positively polarised hydrogens in esters. Remember ethyl butyrate, for instance, which smells like pineapple.
Esterification Of Carboxylic Acids With Alcohols
The classic synthesis is the Fischer esterification, which involves treating a carboxylic acid with an alcohol in the presence of a dehydrating agent:
- RCO O }}
The equilibrium constant for such reactions is about 5 for typical esters, e.g., ethyl acetate. The reaction is slow in the absence of a catalyst. Sulfuric acid is a typical catalyst for this reaction. Many other acids are also used such as polymeric sulfonic acids. Since esterification is highly reversible, the yield of the ester can be improved using Le Chatelier’s principle:
- Using the alcohol in large excess .
- Using a dehydrating agent: sulfuric acid not only catalyzes the reaction but sequesters water . Other drying agents such as molecular sieves are also effective.
- Removal of water by physical means such as distillation as a low-boiling azeotropes with toluene, in conjunction with a Dean-Stark apparatus.
Reagents are known that drive the dehydration of mixtures of alcohols and carboxylic acids. One example is the Steglich esterification, which is a method of forming esters under mild conditions. The method is popular in peptide synthesis, where the substrates are sensitive to harsh conditions like high heat. DCC is used to activate the carboxylic acid to further reaction. 4-Dimethylaminopyridine is used as an acyl-transfer catalyst.
Another method for the dehydration of mixtures of alcohols and carboxylic acids is the Mitsunobu reaction:
- RCO
- H }}
Don’t Miss: What Does Anticyclone Mean In Geography
Difference Between Ester And Ether
Ester and Ether are functional classes that are used to classify organic chemical compounds. Esters are prepared via the process of esterification. The main difference between ether and ester lies in their structure. An ester group requires two oxygen atoms and two carbon atoms to complete its characteristic structure, while an ether group only needs one oxygen atom and two carbon atoms for its structure. The various other differences between ether and ester are given below in a tabular column.
Key Concepts And Summary
Functional groups related to the carbonyl group include the CHO group of an aldehyde, the CO group of a ketone, the CO2H group of a carboxylic acid, and the CO2R group of an ester. The carbonyl group, a carbon-oxygen double bond, is the key structure in these classes of organic molecules: Aldehydes contain at least one hydrogen atom attached to the carbonyl carbon atom, ketones contain two carbon groups attached to the carbonyl carbon atom, carboxylic acids contain a hydroxyl group attached to the carbonyl carbon atom, and esters contain an oxygen atom attached to another carbon group connected to the carbonyl carbon atom. All of these compounds contain oxidized carbon atoms relative to the carbon atom of an alcohol group.
Don’t Miss: What Is Metathesis In Chemistry
Examples In Alcoholic Beverages
The natural esterification that takes place in wines and other alcoholic beverages during the aging process is an example of acid-catalysed esterification. Over time, the acidity of the acetic acid and tannins in an aging wine will catalytically protonate other organic acids , encouraging ethanol to react as a nucleophile. As a result, ethyl acetatethe ester of ethanol and acetic acidis the most abundant ester in wines. Other combinations of organic alcohols and organic acids lead to a variety of different esters in wines, contributing to their different flavours, smells and tastes. Of course, when compared to sulfuric acid conditions, the acid conditions in a wine are mild, so yield is low and take years for ester to accumulate.
Effect Of Alcohol To Oil Ratio
FAME yield is significantly influenced by the molar ratio of methanol to oil. According to Hameed et al. high methanol to oil ratio increased the FAME yield at high catalyst loading. The study also added that when methanol was added in excess, it could promote the esterification or transesterification process forward and could also extract products such as glycerin and methyl esters from the system to renew the catalyst surface. This can be seen in Fig. 2.5 where higher methanol to oil ratio compensate for the increase in FAME yield with higher catalyst loading. However, an increase in methanol to oil ratio, approximately 13:1, could decrease the FAME yield. Moreover, dilution of the oil occurs when a large amount of methanol was present and hinders the FAME yield .
FIGURE 2.5. Effect of methanol to oil molar ratio and catalyst loading in a three-dimensional response surface plot of methyl ester yield. Cited with permission from .
Robert J. Ouellette, J. David Rawn, in, 2015
Don’t Miss: Math Caching Algebra 1 Answers
What Is The Name Origin Of Ester
Ester is quite a random nomenclature for a compound derived from a parent acid and parent alcohol.
Is there any reasoning behind using the word ‘ester’ to name such compound ?
One explanation for such nomenclature I found is quoted below from this website
The word “ester” was coined in 1848 by German chemist Leopold Gmelin,probably as a contraction of the German Essigäther, meaning aceticether.
But even then, how does ‘acetic ether’ has to do with anything about ester being a nomenclature for a compound derived from a parent acid and parent alcohol?
- 1$\begingroup$The thing you have to remember is that most of the ‘simple’ compounds and functional groups were discovered before the structures were figured out. The names, therefore, would have been descriptive based on some quirk of the chemical. As musicians and coders know, naming things can be hard. You look at the etymology of most early elements and compounds, the names tend to work out to ‘smells bad’ or ‘green flame’ or ‘oil from Tolu’$\endgroup$
The term “Essigäther” is actually the German name for ethyl acetate i.e Essig = “vinegar” + Äther = “ether”. “vinegar” becomes acetic, hence ethyl acetate becomes “acetic ether”.
But, ethyl acetate is an ester. So, why is it named “acetic ether”?
According to Leopold Gmelin, ester was called “oxy-acids ether” or “ether of third type”. The original German text is:
which translates to:
Other compound classes originally named ethers
Naphthas referred to