How Is Malleability Determined
In other words, it is the property of a metal to deform under compression and take on a new shape. A metals malleability can be measured by how much pressure it can withstand without breaking. Differences in malleability among different metals are due to variances in their crystal structures.
What Types Of Substances Are Malleable
Definition of Malleable A common example of a malleable material is gold, which is often compressed into gold leaf for use in art, architecture, jewelry and even food. Other malleable metals include iron, copper, aluminum, silver and lead, as well as the transition metal zinc at certain temperatures.
What Is Malleability In Metal
- University of British Columbia
- Carleton University
Terence Bell is a former writer who has been involved in the rare earth and minor metal industries for over 10 years.
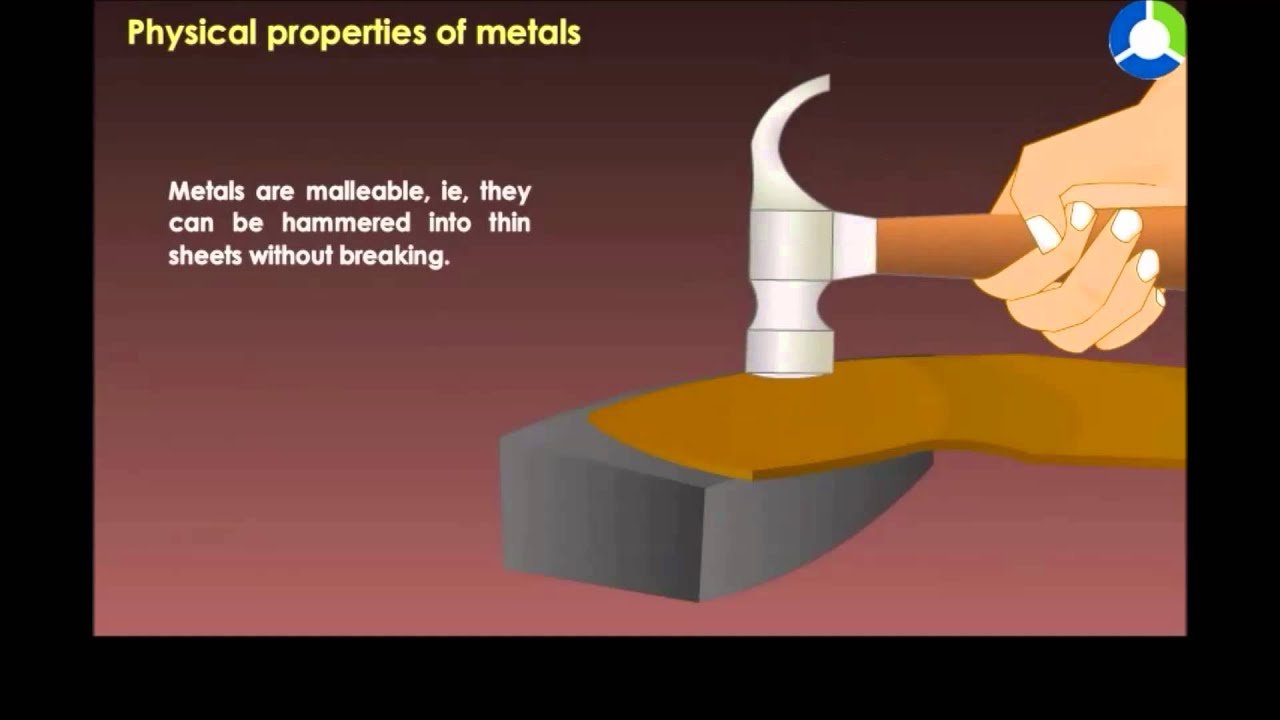
Malleability is a physical property of metals that defines their ability to be hammered, pressed, or rolled into thin sheets without breaking. In other words, it is the property of a metal to deform under compression and take on a new shape.
A metal’s malleability can be measured by how much pressure it can withstand without breaking. Differences in malleability among different metals are due to variances in their crystal structures.
Recommended Reading: My Hrw Com Algebra 1
What Are Malleable Metals
Malleability is a substances ability to deform under pressure . Examples of malleable metals are gold, iron, aluminum, copper, silver, and lead. Gold and silver are highly malleable. When a piece of hot iron is hammered it takes the shape of a sheet.
Then, What does ductility mean?
Ductility is a measure of a metals ability to withstand tensile stressany force that pulls the two ends of an object away from each other. The term ductile literally means that a metal substance is capable of being stretched into a thin wire without becoming weaker or more brittle in the process.
Considering this, What does malleable mean in science? The reason metals bend or dent when struck is linked to malleability, a physical property that is very important to chemists as well as engineers. Malleability is the ability of a substance, usually a metal, to be deformed or molded into a different shape.
28 Related Questions and Answers Found ?
Effect Of Temperature On Malleability

In most metals, increasing temperature reduces the number of grain boundaries and increases malleability. So, some metals that arent malleable under ordinary conditions respond to heat treatment. For example, zinc is brittle until its heated above 300 °F . Above this temperature, its possible to roll the metal into sheets.
Don’t Miss: Who Are Paris Jackson’s Biological Parents
Are Any Nonmetals Malleable
Generally speaking, the elements that are nonmetals are not malleable. However, there are a few exceptions. Certain allotropes are malleable. An example is the plastic allotrope of sulfur.
While nonmetallic elements are not malleable, some nonmetallic polymers are malleable. For example, some plastics display malleability.
Examples Of Malleable In A Sentence
Wall Street JournalNational Geographic TravelerThe Evidence of Things Not Seenmalleablemalleable oregonlivemalleablealmalleable TimemalleableForbesmalleableGlamourmalleable SELFmalleable EW.commalleable Star Tribune
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘malleable.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
You May Like: Who Are Paris Jackson’s Biological Parents
Choose The Right Synonym For Malleable
plastic, pliable, pliant, ductile, malleable, adaptable mean susceptible of being modified in form or nature. plastic applies to substances soft enough to be molded yet capable of hardening into the desired fixed form. plastic materials allow the sculptor greater freedompliable suggests something easily bent, folded, twisted, or manipulated. pliable rubber tubingpliant may stress flexibility and sometimes connote springiness. an athletic shoe with a pliant sole ductile applies to what can be drawn out or extended with ease. ductile metals such as coppermalleable applies to what may be pressed or beaten into shape. the malleable properties of gold adaptable implies the capability of being easily modified to suit other conditions, needs, or uses. computer hardware that is adaptable
What Is Meant By Malleability And Ductility
Malleability and ductility are related. A malleable material is one in which a thin sheet can be easily formed by hammering or rolling. In other words, the material has the ability to deform under compressive stress. In contrast, ductility is the ability of a solid material to deform under tensile stress.
You May Like: Grade 6 Fsa Warm Ups Answer Key
Explain The Meaning Of Malleable And Ductile
Some of the more important properties peculiar to solids are malleability and ductility.
- Ductility is the property of being drawn into wire. It is a permanent strain that accompanied fracture in a tension test. It is a desirable property in machine components that are subjected to unanticipated overloads or impact loads. The ductility of the metals decreases as the temperature increases because metals become weak at increasing temperature. All ductile materials are also malleable, however, the converse is not always true.
- Malleability is defined as the ability of the material to deform to a greater extent before signs of cracks appear when it is subjected to a compressive force. The term malleability comes from the word hammer and in a narrow sense, means forged or extruded because these processes involve shaping under compressive force. In general malleability increases with an increase in temperature. Therefore, processes like forging or rolling are hot working processes where hot ingots or slabs are given shapes.
Was this answer helpful?
Physical Properties Can Be Extensive Or Intensive
What are Extensive Properties?
Extensive Physical Properties are those that depend on the “extent” of the system. Volume and mass are extensive, and two gallons of water at 20 deg C have twice the volume and mass as one gallon of water at 20 deg C.
What are Intensive Properties?
Intensive physical properties do not depend on the “extent” of the system. Density and temperature are intensive, when you combine 2 gallons of water the temperature stays at 20 deg and the density stays at approximately 1g/ml. Intensive properties are often constants and can be used to identify a substance.
Table\: Common Physical Properties
| Texture |
Don’t Miss: What Is The Molecular Geometry Of Ccl4
What Is Material Give Example
An example of material is the wood used to build something. Of, relating to, or composed of matter. The definition of material refers to a physical object, as opposed to something spiritual or mental, or something that is essential and relevant. An example of material is an interest in the physical space around you.
Is Africa A Homogeneous

Critically, from a business perspective, Africa is not a monolith, African countries are not homogeneous, and focusing on Africa as a unit of analysis is, simply put, not terribly meaningful. … And, of course, each country’s economy has a unique character, making it wholly a misnomer to refer to the African economy.
You May Like: Algebra 1 Age Word Problems
What Does Ductile Mean In Science
: the quality or state of being ductile especially: the ability of a material to have its shape changed without losing strength or breaking When certain alloys are added to metal, hardness and strength can be improved without decreasing the ductility.
Why Are Metals Malleable
Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal.
Explanation:
Metallic bonds involve all of the metal atoms in a piece of metal sharing all of their valence electrons with delocalized bonds.
This is different from ionic bonding and covalent bonding .
A metal that you can hammer into thin sheets is malleable.
Gold, silver, aluminum, iron, and copper are malleable.
Non-malleable metals such as tin will break apart when struck by a hammer.
A metal behaves as an array of metal ions or kernels immersed in a sea of mobile valence electrons.
Metallic bonds consist of the attractions of the ions to the surrounding electrons. Metallic bonds are non-directional.
Whenever a metal receives a stress, the position of adjacent layers of metallic kernels shifts.
The atoms roll over each other but the environment of the kernels does not change.
The deforming force just moves the kernels from one lattice site to another.
Read Also: Does Mj Have Any Biological Kids
Does Malleable Mean Flexible
is that malleable is able to be hammered into thin sheets capable of being extended or shaped by beating with a hammer, or by the pressure of rollers while flexible is capable of being flexed or bent without breaking able to be turned, bowed, or twisted, without breaking pliable not stiff or brittle.
What Is Meant By Ductility
Ductilityductileductile
Bryon Henon
Mirjana Glassmacher
malleable Sentence Examples
Douaa Raposo
Opposite
Xue Baldes
Synonyms
Haroun Agnivtsev
Recommended Reading: Segment Addition Postulate Kuta
Controlling Crystal Grains Through Temperature
Temperature has a direct effect on the behavior of atoms, and in most metals, heat results in atoms having a more regular arrangement. This reduces the number of grain boundaries, thereby making the metal softer or more malleable.
An example of temperature’s effect on metals can be seen with zinc, which is a brittle metal below 300 degrees Fahrenheit . However, when it’s heated above this temperature, zinc can become so malleable it can be rolled into sheets.
Cold working stands in contrast to heat treatment. This process involves rolling, drawing, or pressing a cold metal. It tends to result in smaller grains, making the metal harder.
Beyond temperature, alloying is another common method of controlling grain sizes to make metals more workable. Brass, an alloy of copper and zinc, is harder than both individual metals because its grain structure is more resistant to compression stress.
What Does Malleability Mean
Malleability describes the property of a metal’s ability to be distorted below compression. It is a physical property of metals by which they can be hammered, shaped and rolled into a very thin sheet without rupturing. A malleable fabric could be planate by blow or rolling.
Malleability in metals are very important in the appliance and automotive industries. This property helps to construct refrigerators, microwaves and stoves, and also helps to construct flat and curved metal objects.
Read Also: What Is An Inertial Frame Of Reference In Physics
Melting Point And Boiling Point
When a metal melts or boils, this is a change of physical state.
is transferred to a substance to melt or boil it. This energy is needed to overcome the forces of attraction between the metal ions in the metal. The more energy needed, the higher the melting point or boiling point.
As metals are giant lattice structures, the number of electrostatic forces to be broken is extremely large, and so metals have high melting and boiling points. This means that the melting point and boiling point of metals are more similar to those for ionic compounds
Difference Between Malleable And Ductile

A malleable material deforms under compression, while a ductile material deforms under tensile stress. Malleable metals are beaten or pressed into thin sheets, while ductile metals are drawn into thin wires.
For the most part, malleability and ductility go hand in hand. However, there are some metals that are malleable, yet not ductile. For example, lead is malleable and can be pressed into thin sheets. Yet, it is not ductile and easily fractures when drawn into wires.
Read Also: What Is The Molecular Geometry Of Ccl4
Metallic Structure And Bonding
Most of the elements in the periodic table are metals. Properties of metals can be explained in terms of metallic structure and bonding.
Some metals have properties that are not typical. For example:
- mercury has a low melting point and exists as a liquid at room temperature
- have low melting points, but also low densities, for example, sodium is less dense than water and so it floats
A substance with a high density means it has a high mass for its size. For example, the volume of liquid in a can of drink is 330 cm3. If the can was filled with sodium then the can would have a mass of around 320 g. However, if the can was filled with lead , then the can would have a mass of nearly 3.75 kg!
Malleable substances can be bent or hammered into shape without shattering, while brittle substances shatter when bent or hit.
Metals are described as ductile because they can be drawn out into thin wires.
What Is The Opposite Of Malleable In Science
. Likewise, people ask, what is the opposite of malleable and ductile?
Brittle is the property of the non metal which says them to be non malleable and non ductile.
Subsequently, question is, what is the definition of malleable in science? The reason metals bend or dent when struck is linked to malleability, a physical property that is very important to chemists as well as engineers. Malleability is the ability of a substance, usually a metal, to be deformed or molded into a different shape.
Keeping this in consideration, what is the synonym of malleable?
Synonyms: bendable, flexible, tractile, elastic, pliant, ductile, plastic, waxy, tensile, fictile, pliable. Antonyms: intractable, unformed.
What is not malleable?
Some of the examples of malleable metal includes of aluminum, silver, lead, copper, iron, gold and many others. Mercury is not malleable at room temperature since it is liquid. Malleability is one of the significant properties of metal.
Read Also: What Does Abiotic
What Is The Definition Of Malleable In Science
4.4/5Malleabilitymalleabilitymeans
People also ask, what is the definition of malleability?
malleability. Malleability is the quality of something that can be shaped into something else without breaking, like the malleability of clay. Malleability also called plasticity has to do with whether something can be molded.
Similarly, what is the opposite of malleable in science? Brittle is the property of the non metal which says them to be non malleable and non ductile.
One may also ask, what is an example of malleable?
It is the ability of a solid to bend or be hammered into other shapes without breaking. Examples of malleable metals are gold, iron, aluminum, copper, silver, and lead. Gold and silver are highly malleable. When a piece of hot iron is hammered it takes the shape of a sheet.
What does malleable mean for kids?
Malleability is a substance’s ability to deform under pressure . If malleable, a material may be flattened into thin sheets by hammering or rolling. Malleability is a physical property of matter, usually metals.
What Are Tools And Equipment
Tools and Equipment means all hand tools, implements, camp equipment, drawing office and survey instruments, medical and surgical instruments and all articles of similar nature, whether or not they are of an expendable nature, which are not normally issued to officers personally for use in carrying out their official
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
What Is Heterogeneous Culture
Definition. “Cultural heterogeneity refers to differences in cultural identity related to, for instance, class, ethnicity, language, traditions, religion, sense of place, and many other cultural aspects. These differences can make it more or less difficult for people to communicate, trust and co-operate with each-other …
What Do You Mean By Plasticity
Plasticity means changeability or moldability clay has a lot of plasticity, but a rock has almost none. Plasticity refers to things that can still change their shape or function. The brain is something with high plasticity: if you have a brain injury, other parts of the brain can change to pick up the slack.
Recommended Reading: What Does Abiotic Mean In Biology
What Makes Metals So Marvelous
The 2019 National Chemistry Week theme is Marvelous Metals, and no wonder. These ubiquitous elements, which make up more than 75% of the periodic table, are everywhere doing amazing things. The cobalt in vitamin B12 helps us make red blood cells, the iron in hemoglobin carries oxygen to tissues, and sodium and potassium ions allow the heart and nerves to communicate.
Want to get from city to city or around the world without metalsforget it. Aluminum makes aviation possible, and steel forms the frames of cars, buses, and trains. Not to mention the copper, silicon, and rare-earth metals that make all of our electrical power, computers, and smart devices possible.
And the possibilities keep growing. From gold nanoparticles that can detect and treat cancer to vanadium-stabilized batteries to aluminum-based materials that can harvest water from desert air, metals are humanitys past, present, and future.
You already know some technical basics about metals. They are shiny, malleable, fusible, ductile, good conductors of electricity and heat, and inclined to give up electrons to form cations. But do you know why? What makes metals so marvelous? The answer, of course, lies at the atomic scale.
Can You Guess What Types Of Metals These Screws Are Made Of
Screws come in all sizes and shapes. They are all made of some kind of metal. But they have differences in size, shape, and type of metal. Physical characteristics also differ. Some screws are long, and others are short. One screw may have a flat-head slot while another screw may have a Phillips-head. Some of the screws in the picture below are used to fasten things together, and others are used to hang heavy objects on a wall.
Chemists classify materials in many ways. We can sort elements on the basis of their electron arrangements. The way the electrons are distributed determines the chemical properties of the element. Another way is to classify elements based on physical properties. Some common physical properties are color, volume, and density. Other properties that allow us to sort on the basis of behavior are conduction of heat and electricity, malleability , ductility , melting point, and boiling point. Three broad classes of elements based on physical properties are metals, nonmetals, and metalloids.
Gold has been used by many civilizations for making jewelry . This metal is soft and easily shaped into a variety of items. Since gold is very valuable and often used as currency, gold jewelry has also often represented wealth.
Copper is a good conductor of electricity and is very flexible and ductile. This metal is widely used to conduct electric current in a variety of appliances, from lamps to stereo systems to complex electronic devices .
|
Figure 1. Gold jewelry. |
Read Also: Kendall Hunt Geometry Answer Key