Examples Of Irreversible Inhibitors
Diisopropylfluorophosphate is shown as an example of an irreversible protease inhibitor in the figure above right. The enzyme hydrolyses the phosphorusfluorine bond, but the phosphate residue remains bound to the serine in the active site, deactivating it. Similarly, DFP also reacts with the active site of acetylcholine esterase in the synapses of neurons, and consequently is a potent neurotoxin, with a lethal dose of less than 100 mg.
Suicide inhibition is an unusual type of irreversible inhibition where the enzyme converts the inhibitor into a reactive form in its active site. An example is the inhibitor of polyamine biosynthesis, -difluoromethylornithine or DFMO, which is an analogue of the amino acid ornithine, and is used to treat African trypanosomiasis . Ornithine decarboxylase can catalyse the decarboxylation of DFMO instead of ornithine, as shown above. However, this decarboxylation reaction is followed by the elimination of a fluorine atom, which converts this catalytic intermediate into a conjugated imine, a highly electrophilic species. This reactive form of DFMO then reacts with either a cysteine or lysine residue in the active site to irreversibly inactivate the enzyme.
Commonly Used Protease And Phosphatase Inhibitors
| Inhibitor |
|---|
We offer a variety of both individual protease inhibitors and ready-to-use, broad-spectrum protease and phosphatase inhibitor cocktails. The inhibitor cocktails are available as both 100X cocktail solutions and quick-dissolving tablets to accommodate specific and general needs in cell lysis and protein extraction methods. These inhibitors are suitable for the protection of proteins during their extraction from cultured cells, animal tissues, plant tissues, yeast or bacteria.
Our Thermo Scientific protease inhibitor cocktails and tablets target serine-, cysteine- and aspartic acid proteases and aminopeptidases. Metalloproteases are inhibited by the optional addition of EDTA . Our Thermo Scientific phosphatase inhibitor cocktails and tablets contain chemical compounds that target serine/threonine and tyrosine phosphatases.
Also available are combined Thermo Scientific protease and phosphatase inhibitor cocktails and tablets. These prevent protein degradation and preserve phosphorylation simultaneously, providing complete protection in a single solution or tablet.
All Halt inhibitor cocktails and Pierce inhibitor tablets are compatible with Thermo Scientific Pierce protein extraction reagents and most homemade and commercial cell lysis solutions.
Antiviral Activity Of Ac
The human hepatoma cell line Huh7 was maintained in DMEM supplemented with 10% fetal bovine serum , 2% HEPES 1M , 5ml of sodium bicarbonate 7.5% 1% nonessential amino acids and 1% penicillin-streptomycin 10,000Uml1 in a humidified 5% CO2 incubator at 37°C. Assay medium, used for producing virus stocks and antiviral testing, was prepared by supplementing DMEM with 4% FBS, 2% HEPES 1M, 5ml of sodium bicarbonate 7.5 and 1% NEAA.
To quantify antiviral activity on Huh7 cells, we selected a SARS-CoV-2 virus strain that produces sufficient cytopathogenic effect on this cell line. We started from passage 6 of the SARS-CoV-2 strain BetaCov/Belgium/GHB-03021/2020 that has been described previously, and passaged this three additional times on Huh7 cells while selecting those cultures that showed most CPE. This resulted in a virus stock that confers full CPE on Huh7 per ml) as well as on VeroE6 cells . The genotype of this virus stock shows four nucleotide changes as compared with the mother virus stock and these are currently being analyzed. None of the nucleotide changes occur in the part of the genome that encodes the 3C-like protease, validating this virus stock for testing protease inhibitors.
All virus-related work was conducted in the high-containment BSL3+ facilities of the KU Leuven Rega Institute under licenses AMV 30112018 SBB 219 2018 0892 and AMV 23102017 SBB 219 2017 0589 according to institutional guidelines.
You May Like: How To Get Moles Chemistry
Can Capsaicin Kill Bacteria
Capsaicin works to kill bacteria when consumed within food, such as in raw oysters. Research has found that when cayenne or other types of chile is consumed with noxious bacteria in oysters, all of the bacteria are then killed. Externally applied, capsaicin compounds work to relieve sore muscles and joints.
Does The Zone Of Inhibition Edge Indicate
For best results enter two or more search terms.MICRO.
| Question | Answer |
|---|---|
| does the zone of inhibitions edge indicate the limit of antibacterial agent diffusion into the agar? | No. edge is no the unit of diffusion. diffusion occurs beyond edge, but concentration of Bactraicin is to dilute to be lethal -the edge represent MIC |
Recommended Reading: What Math Is Needed For Finance
Superimposing Crystal Structures For Retaining Partial Molecular Inhibitor Fragments
Having a crystal structure of an enzyme-substrate complex is possible in case a catalytic active residue has been mutated or in case of a slow substrate. In case of a mutation, it is not obvious that the substrate resides in exactly the same position as it would have been in the native enzyme, which is not amenable to crystal structure analysis due to the fast reaction. However, a POP structure with a catalytic residue mutation S554A is the starting point for all our current study of POP.
To avoid the blocking of peptide cleavage, we begin by superimposing the crystal structure of the inhibitor complex over the structure of the complex with the substrate. That, in order to identify which inhibitor fragments are equivalent to P1-P1′ of the substrate cleavage positions, that reside in the protein pockets S1-S1′. Those inhibitor fragments should be rejected so as to supply partial structures that do not intervene directly with the cleavage. Other molecular parts between S1-S1′ and the N-terminal are retained for subsequent modeling steps. To identify those inhibitor fragments, we superimposed the single POP-substrate complex over a set of 15 POP-inhibitor complexes. In Fig 2A we present the original substrate position next to the catalytic triad of POP while Fig 2B shows the computeraligned conformation of an inhibitor.
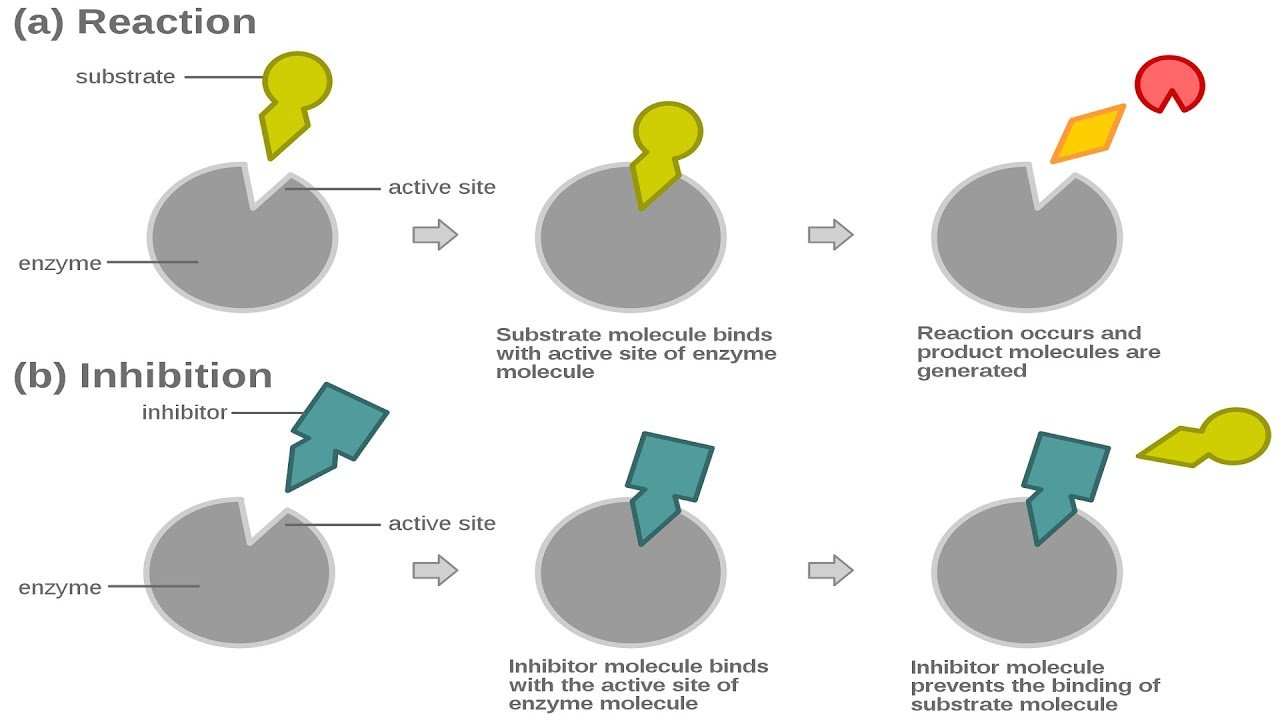
What Is Enzyme Inhibition
Enzyme inhibition is a reaction between a molecule and an enzyme that blocks the action of the enzyme, either temporarily or permanently, depending on the type of enzyme inhibitor involved. This process occurs in the natural world all the time, and it has a number of applications for humans, including in the formulation of pharmaceuticals and the development of certain products. There are several types of enzyme inhibition involving different types of molecules and processes.
Non-specific enzyme inhibition involves exposure to a molecule not specifically targeting an enzyme that will still have an inhibitory effect. For example, many proteins break down in response to alcohol exposure, and introducing alcohol to an organism can inhibit the activities of a number of enzymes. Acids have similar effects. Likewise, heat tends to denature enzymes, pulling the protein strands apart and rendering them ineffective this is why meat softens with cooking, as the proteins in the meat are denatured and start to break down.
Read Also: What Does Concentration Mean In Geography
Examples Of Inhibition In A Sentence
inhibitioninhibitioninhibitionForbesinhibition The New York Review of Booksinhibition Smithsonian MagazineinhibitionForbesinhibition Billboardinhibition Washington Postinhibition ForbesinhibitionForbes
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘inhibition.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Evidence For The Physical Continuity Of Soluble And Nerve Ending Tryptophan Hydroxylase
Additional evidence was sought for the cell body location of the soluble enzyme, for the nerve ending location of the particulate enzyme, and the relationship between them. This was done using the strategy of the acute administration of the tryptophan hydroxylase inhibitor parachlorophenylalanine and following the flow of reduced activity from the midbrain region to the septal areas. Due to the multiplicity of actions of PCPA, both in vitro and in vivo experiments were carried out. Figure 3 demonstrates that PCPA is a competitive inhibitor of uptake of labeled tryptophan into synaptosomes with a Ki of 9.7 × 106 M. Other work has demonstrated a dialyzable competitive and non-dialyzable non-competitive inhibition of soluble enzyme by PCPA several hours after administration . In a series of in vivo experiments, PCPA was administered and groups of animals were sacrificed after varying periods of time. Tryptophan hydroxylase activity was determined in the midbrain and septal areas. Figure 4 summarizes the results. The midbrain manifests a decreased activity during the first few hours and a delayed decrease in two days which returned to control levels between eight and thirteen days after drug treatment. The septal area manifested a more immediate decrease, reversible in four hours, and a delayed decrease reaching the septal area in eight to thirteen days after drug administration.
Ivar Roots, … Santosh Nigam, in, 1980
Recommended Reading: How To Calculate Heat Change In Chemistry
Experimental Testing And Analysis Of The Results
Preparation of recombinant human POP is described in the Experimental procedures. The obtained rhPOP showed no enzyme activity other than the substrate specificity of POP for Ang-III and TRH . The chromatograms obtained by analysing the reaction mixture of rhPOP with Ang-III or TRH without inhibitor by RP-HPLC are shown in S6 Fig. In S6A, the peak at RT 11.0 min was that of Phe at the C terminus of Ang-III. In addition, it was confirmed by the protein sequencer that the peak at RT 15.8 min was that of Ang-III fragment . In S6B Fig, the peak at RT 17.0 min was determined to be that of TRH fragment by MALDI-TOF MS analysis .
First, the effect of 100 M of inhibitor on the POP catalytic action toward Ang-III and TRH was investigated for 20 candidates and 5 decoys for confirmation . Two of the candidates were found to affect differently the two substrates of POP . In presence of these inhibitors, catalytic activity was inhibited between 1075%. One of the two, T6816369, succeeded in all 4 “structure based” pharmacophore tests and the other, T5450157, was successful in the “ligand based” ISE candidate set, with the 2nd highest MBI of 0.971.
Dixon plots of: A) Ang-III cleavage in presence of T6816369 B) TRH cleavage in presence of T6816369 C) Ang-III cleavage in presence of T5450157 D) TRH cleavage in presence of T5450157.
Allosteric Inhibition Prevents The Over
Allosteric inhibitors prevent the body from wasting energy to create unnecessary products. Think of a metabolic pathway as an assembly line at a factory. At each station in the assembly line, a machine alters the product before passing it to the next station. The assembly line then moves intermediate products from station to station until there is a final product at the end.
Say that this factory is responsible for producing pants. At the first station, a machine cuts a pair of pants from raw material. Subsequently, a machine at the second station stitches the hems of the pants together. At the third station, a machine attaches the zippers. After that, a machine at the fourth station attaches tags and places the product in the shipment pile. When the machines are working properly, the assembly line is smooth and there is no hold-up. Stations will produce products at similar rates.
However, imagine that one day, the machine that attaches the zippers at the third station breaks down. There is now a hold-up. To prevent products from piling up, we must stop the first few stations of the supply line from operating. This is to control the supply and demand for each intermediate product and to ensure that they are equal at each station.
Similarly, allosteric inhibition slows down the chain of reactions. In this way, it prevents the over-accumulation of unnecessary products.
Don’t Miss: What Is 9th Grade Math
What Spices Have Antibacterial Properties
Many spicessuch as clove, oregano, thyme, cinnamon, and cuminpossessed significant antibacterial and antifungal activities against food spoilage bacteria like Bacillus subtilis and Pseudomonas fluorescens, pathogens like Staphylococcus aureus and Vibrio parahaemolyticus, harmful fungi like Aspergillus flavus, even
Why Do Susceptibility Breakpoints Differ For Different Antibiotics
These differences may result from different dosing of drugs in various countries or from use of different laboratory methods to determine antibiotic susceptibility. In addition, some philosophical differences may exist among the various organizations and societies responsible for issuing these breakpoints.
Read Also: What Is Cro In Physics
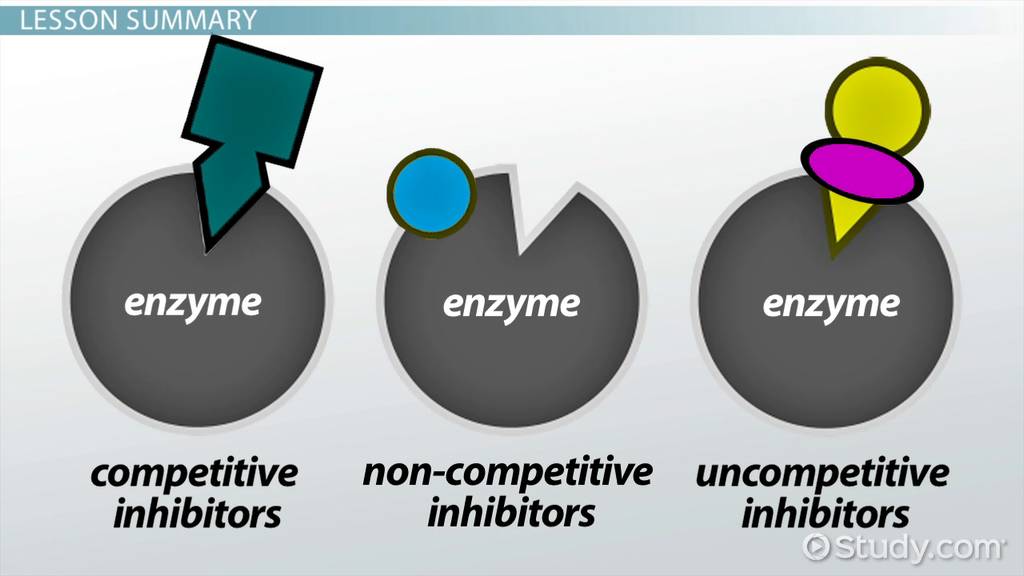
Quantitative Description Of Reversible Inhibition
Reversible inhibition can be described quantitatively in terms of the inhibitor’s binding to the enzyme and to the enzyme-substrate complex, and its effects on the kinetic constants of the enzyme. In the classic Michaelis-Menten scheme below, an enzyme binds to its substrate to form the enzymesubstrate complex ES. Upon catalysis, this complex breaks down to release product P and free enzyme. The inhibitor can bind to either E or ES with the dissociation constantsKi or Ki’, respectively.
|
Kinetic scheme for reversible enzyme inhibitors |
Do We Discover Better Than Random
Several methods could be used for discovering SSI. The most prominent one is probably massive docking limited by the specific requirements of inhibition. That may be an extremely lengthy process with unclear outcome. In our case, we limited our search for inhibiting one out of two substrates. Along the discovery process, we reduced the number of possible candidates to those that include appropriate fragments, and achieved that by “trimming” known inhibitors to the scaffolds that could serve the discovery. In the final step, we screened only those ~12,000 molecules, a very small number out of the original ~1.8 million in the library, to reach the final candidates.
Read Also: What Is Gradualism In Biology
Clinical Characteristics Of One Patient Recruited At Cuhl
P01, a 13-year old boy, presented with mild symptoms of COVID-19 infection and no need for respiration support or mechanical ventilation no specific treatment was applied. Two nasopharyngeal swabs for Mpro activity were collected during routine clinical examination, at days 1 and 5 after COVID-19 diagnosis. Tests positive for SARS-CoV-2 RNA were obtained on days 1 and 5, the first negative test was on day 7.
What Is Feedback Inhibition And How Does It Work
In biochemistry, metabolic pathways are activated and regulated in a variety of ways. One such method involves the inhibition of the entire pathway by the increased concentration of the end product. This process is called feedback inhibition, and is explained in this BiologyWise post.
Like it? Share it!
In biochemistry, metabolic pathways are activated and regulated in a variety of ways. One such method involves the inhibition of the entire pathway by the increased concentration of the end product. This process is called feedback inhibition, and is explained in this BiologyWise post.
Recommended Reading: What Is An Example Of Movement In Geography
Enzyme Inhibitors As Pharmaceutical Agents
We have mentioned above nonsteroidal anti-inflammatory drugs that are the inhibitors of cyclooxygenase. This group of compounds was successfully used for more than one century around the whole world for treatment of patients with fever, cardiovascular diseases, joint pain, etc. . Among these drugs are both irreversible and reversible inhibitors that slow down production of prostaglandins that control many aspects of inflammation, smooth muscle contraction, and blood clotting. But there are many other groups of drugs that are by nature of inhibitors of some enzymes the following groups of enzyme inhibitors are developed now by pharmaceutical companies and have very important therapeutic significances .
Inhibitors of angiotensin-converting enzyme . ACE catalyzes a conversion of inactive decapeptide angiotensin I into angiotensin II by the removal of a dipeptide from the C-terminus of angiotensin I. Angiotensin II is a powerful vasoconstrictor. Inhibition of ACE results in the decrease of angiotensin I concentration and in the relaxation of smooth muscles of vessels. Inhibitors of ACE are widely used as drugs for treatment of arterial hypertension .
Antibiotic penicillin covalently modifies the enzyme transpeptidase, thereby preventing the synthesis of bacterial cell walls and thus killing the bacteria .
Measuring The Dissociation Constants Of A Reversible Inhibitor
As noted above, an enzyme inhibitor is characterised by its two dissociation constants, Ki and Ki’, to the enzyme and to the enzyme-substrate complex, respectively. The enzyme-inhibitor constant Ki can be measured directly by various methods one extremely accurate method is isothermal titration calorimetry, in which the inhibitor is titrated into a solution of enzyme and the heat released or absorbed is measured. However, the other dissociation constant Ki’ is difficult to measure directly, since the enzyme-substrate complex is short-lived and undergoing a chemical reaction to form the product. Hence, Ki’ is usually measured indirectly, by observing the enzyme activity under various substrate and inhibitor concentrations, and fitting the data to a modified MichaelisMenten equation
- V
- . =1+^}}.}
Thus, in the presence of the inhibitor, the enzyme’s effective Km and Vmax become Km and Vmax, respectively. However, the modified Michaelis-Menten equation assumes that binding of the inhibitor to the enzyme has reached equilibrium, which may be a very slow process for inhibitors with sub-nanomolar dissociation constants. In these cases, it is usually more practical to treat the tight-binding inhibitor as an irreversible inhibitor however, it can still be possible to estimate Ki’ kinetically if Ki is measured independently.
You May Like: What Is Psychological Safety At Work