How Does Using Moles Help Scientists
Atoms and molecules are much smaller than sprinkles. The mole exists to help scientists work with such tiny particles.;
For example, diamonds are made of carbon. A mole of carbon atoms has a mass of about 12 grams. The Tiffany Diamond, one of the largest diamonds in the world, weighs about 26 grams. This is more than two moles of carbon. So, a mole of atoms is a straightforward quantity for people to measure.
The number of particles in a mole, 6.022×1023, is often called Avogadro’s constant or Avogadros number. It is named after Amedeo Avogadro, an Italian physicist.
So when would anyone use Avogadro’s constant? Likely when making a solution that involves many chemical reactions. When performing a reaction, you combine appropriate numbers of molecules of each chemical based on the number of moles required.
To determine how much of a solution to use, chemists use molarity. Thats the number of moles of a chemical in a litre of solution.
A concentration of 1 mole in 1 litre is called 1 molar and abbreviated 1 M or 1 mol/L.
To see this in action, lets imagine you are a chemist trying to make soap. You need to react vegetable oil or animal fat with sodium hydroxide to produce soap. Imagine you had measured some oil and determined it contained 0.2 moles of fat molecules called triglycerides.
Step One: Calculate moles of sodium hydroxide needed
Multiply the moles of triglycerides needed by the ratio of reactants :
0.2 mol of triglyceride x / = 0.6 mol of sodium hydroxide
Performing Grams And Moles Conversions
Here are some tips for performing these conversions:
- The two problems most commonly encountered are setting up the problem incorrectly, so the units don’t cancel out and give the correct result. It helps to write out the conversion and make sure units cancel. You may want to draw a line through them in complex calculations to keep track of active units.
- Watch your significant figures. Chemistry professors are unforgiving when it comes to reporting an answer, even if you set up the problem correctly.
How To Convert Moles To Molecules With Examples
To convert moles to molecules you will need to use two equations and have at hand Avagadros number and the number of moles in your end substance. See below for examples and formulas.
6.02 x 1023
602,000,000,000,000,000,000,000
In chemistry courses, youll frequently have to convert moles to molecules or molecules to moles using Avogadros number. To do this, youll want to be familiar with the definitions of both moles and molecules, the relationship between the two concepts, and the exact formula which uses Avogadros number.
You May Like: Geometry Lesson 1.7 Answers
How Is A Mole Calculated
If you want to know how many moles of a material you have, divide the mass of the material by its molar mass. The molar mass of a substance is the mass in grams of one mole of that substance. This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units . For example, silver has an atomic weight of 107.8682 amu, so one mole of silver has a mass of 107.8682 grams.
mole, also spelled mol, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
The mole designates an extremely large number of units, 6.02214076 × 1023. The General Conference on Weights and Measures defined the mole as this number for the International System of Units effective from May 20, 2019. The mole was previously defined as the number of atoms determined experimentally to be found in 12 grams of carbon-12. The number of units in a mole also bears the name Avogadros number, or Avogadros constant, in honour of the Italian physicist Amedeo Avogadro . Avogadro proposed that equal volumes of gases under the same conditions contain the same number of molecules, a hypothesis that proved useful in determining atomic and molecular weights and which led to the concept of the mole.
The Mole And Molar Mass
The International Committee for Weights and Measuresa group that defines the metric systems units of measurement defines one mole as the number of atoms in exactly 12 grams of carbon-12 . Experiments counting the number of 12C atoms in a 12-gram sample have determined that this number is 6.02214076 x 1023. Regardless of whether the substance is 12C, electrons, or gray squirrels, one mole represents the same number of each of these things.
Figure 2
Scientists have then defined the molar mass of a substance as the mass of 6.02214076 x 1023units of that substance. So, the molar mass of gray squirrels is 301,000,000,000,000,000,000,000,000 grams. With squirrels, this is not very useful. However, it is quite useful if we apply it to other substances, especially elements. By standardizing the number of atoms in a sample of an element, we also get a standardized mass for that element that can be used to compare different elements and compounds to one another. 12Cs molar mass is 12 grams, which represents the combined mass of 6.02 x 102312C atoms. However, other elements have different molar masses; for example, 6.02 x 1023 sulfur-32 atoms have a mass together of 31.97 grams, which is 32Ss molar mass.
Comprehension Checkpoint
= Number of sample molecules
To understand how molar mass and Avogadros number act as conversion factors, we can turn to an example using a popular drink: How many CO2molecules are in a standard bottle of carbonated soda?
Figure 3
Equation 3
Also Check: Segment Addition Postulate And Midpoint Worksheet Answer Key
Determine The Molecular Mass Of A Chemical Compound
Determine the molecular mass of carbon dioxide . Find carbon and oxygen on the periodic table.
Note the masses of carbon and oxygen from the periodic table, which are 12.01 and 16, respectively.
Add the mass numbers of one atom of carbon and two atoms of oxygen from the periodic table: 12.01 + 2 = 44.01 grams per mole
How To Calculate Molar Mass
This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.There are 7 references cited in this article, which can be found at the bottom of the page.wikiHow marks an article as reader-approved once it receives enough positive feedback. This article has 13 testimonials from our readers, earning it our reader-approved status. This article has been viewed 1,066,284 times.
Atoms are too small to allow meaningful measurement of chemical substances. To work with meaningful amounts of substances, scientists group them into units called moles. A mole is defined as the number of carbon atoms in 12 grams of the isotope carbon-12,XResearch source which is roughly 6.022 x 1023 atoms. This number is called Avogadro’s number or Avogadro’s constant.XResearch source This constant is used as the number of atoms given by one mole for any substance, and the mass of 1 mole of a substance is its molar mass.
Recommended Reading: Geometry Segment Addition Postulate Worksheet
Converting Moles To Grams
One of the most common chemistry calculations is converting moles of a substance into grams. When you balance equations, you’ll use the mole ratio between reactants and reagents. To do this conversion, all you need is a periodic table or another list of atomic masses.
Example: How many grams of carbon dioxide is 0.2 moles of CO2?
Look up the atomic masses of carbon and oxygen. This is the number of grams per one mole of atoms.
Carbon has 12.01 grams per mole.Oxygen has 16.00 grams per mole.
One molecule of carbon dioxide contains 1 carbon atom and 2 oxygen atoms, so:
number of grams per mole CO2 = 12.01 + number of grams per mole CO2 = 12.01 + 32.00number of grams per mole CO2 = 44.01 gram/mole
Simply multiply this number of grams per mole times the number of moles you have in order to get the final answer:
grams in 0.2 moles of CO2 = 0.2 moles x 44.01 grams/molegrams in 0.2 moles of CO2 = 8.80 grams
It’s good practice to make certain units cancel out to give you the one you need. In this case, the moles canceled out of the calculation, leaving you with grams.
Formula Mass And The Mole Concept
- Calculate formula masses for covalent and ionic compounds
- Define the amount unit mole and the related quantity Avogadroâs numberExplain the relation between mass, moles, and numbers of atoms or molecules, and perform calculations deriving these quantities from one another
Many argue that modern chemical science began when scientists started exploring the quantitative as well as the qualitative aspects of chemistry. For example, Daltonâs atomic theory was an attempt to explain the results of measurements that allowed him to calculate the relative masses of elements combined in various compounds. Understanding the relationship between the masses of atoms and the chemical formulas of compounds allows us to quantitatively describe the composition of substances.
Recommended Reading: What Is The Lewis Dot Structure For Ccl4
Stoichiometry And Balanced Equations
In stoichiometry, balanced equations make it possible to compare different elements through the stoichiometric factor discussed earlier. This is the mole ratio between two factors in a chemical reaction found through the ratio of stoichiometric coefficients. Here is a real world example to show how stoichiometric factors are useful.
Example 2
There are 12 party invitations and 20 stamps. Each party invitation needs 2 stamps to be sent. How many party invitations can be sent?
Solution
What is the limiting reagent in this example?
Solution
Stamps, because there was only enough to send out invitations, whereas there were enough invitations for 12 complete party invitations. Aside from just looking at the problem, the problem can be solved using stoichiometric factors.
12 I x = 12 IS2 possible
20 S x = 10 IS2 possible
When there is no limiting reagent because the ratio of all the reactants caused them to run out at the same time, it is known as stoichiometric proportions.
How Is A Mole Defined
A mole is defined as 6.02214076 × 1023 of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance. The mole was originally defined as the number of atoms in 12 grams of carbon-12, but in 2018 the General Conference on Weights and Measures announced that effective May 20, 2019, the mole would be just 6.02214076 × 1023 of some chemical unit.
You May Like: What Are Dyes
The History Of Avogadros Number:
Though called Avogadros number, the person who originally estimated the number of particles in a certain substance was Josef Loschmidt, who put the value of particles in one cubic centimeter of gas at 2.6867773 x 1025 m-3. The term Avogadros number was coined by a French physicist, Jean Baptiste Perrin. Perrin made an estimate of what he called Avogadros number. Avogadro had been the first physics professor in Italy and had created a hypothesis that suggested gases of equal volume at the same temperature and pressure should contain the same number of particles. Perrin used Loschmidts constant and Avogadros hypothesis to create the Avogadro number.
Amount Of Substance And The Mole

Amount of Substance and the Mole
The International System of Units, the SI, is built upon seven base quantities and seven base units, as summarized in the table below. Although most of these are familiar to all scientists, the quantity amount of substance and its unit mole are less familiar and are mainly used by chemists. In the chemistry community, the unit mole is familiar, but the name of the corresponding quantity amount of substance is not so familiar, and the concept is still a source of difficulty for many students. This article reviews and clarifies these two concepts and discusses the definition of the unit mole and its possible revision.
| Base quantity |
| cd |
Amount of Substance
Amount of substance, symbol n, is a quantity that measures the size of an ensemble of entities. It appears in thermodynamic relations, such as the ideal gas law, and in stoichiometric relations between reacting molecules, as in the Law of Multiple Proportions. Familiar equations involving n are thus
| pV = nRT |
for an ideal gas, and the equation
| c = n/V |
for the amount-of-substance concentration of a solution. Here, V is the volume of a solution containing the amount of solute n. Another important relation is that between amount of substance n and mass m for a pure sample
| n = m/M |
where M is the mass per amount of substance, usually called the molar mass. Similarly, amount concentration c may be related to mass concentration by the equation
| c = /M |
| n = N/NA |
The Mole
| Mu = M / 12 |
Recommended Reading: Geometry Segment Addition Postulate Worksheet
Why We Use Moles
Why don’t we simply stick with units like grams ? The answer is that moles give us a consistent method to convert between atoms/molecules and grams. It’s simply a convenient unit to use when performing calculations. You may not find it too convenient when you are first learning how to use it, but once you become familiar with it, a mole will be as normal a unit as, say, a dozen or a byte.
Scare Moles And Gophers Away
Getting rid of moles and gophers can be a hassle. However, they dont like to live in areas where they are disturbed. A sonic spike inserted into the ground uses electronic pulses to create irritating sounds to drive these pests away. There are multiple ultrasonic pest control options to choose from.;
Some pets will chase gophers away if the gophers see or hear them. Even the scent of a dog or cats fur or urine may drive a gopher away. However, gophers can carry fleas or ticks that transmit diseases, so check your pet carefully if it comes across a gopher and remove or treat for any parasites, following your veterinarian’s instructions.
If you dont own a pet, consider applying a predator scent to your yard, such as coyote urine. This is yet another humane way to drive the pests away, possibly for months. Read and follow the label directions before using the scent.
Read Also: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
Formula Mass For Ionic Compounds
Ionic compounds are composed of discrete cations and anions combined in ratios to yield electrically neutral bulk matter. The formula mass for an ionic compound is calculated in the same way as the formula mass for covalent compounds: by summing the average atomic masses of all the atoms in the compounds formula. Keep in mind, however, that the formula for an ionic compound does not represent the composition of a discrete molecule, so it may not correctly be referred to as the molecular mass.
As an example, consider sodium chloride, NaCl, the chemical name for common table salt. Sodium chloride is an ionic compound composed of sodium cations, Na+, and chloride anions, Cl, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu .
Figure 3.
Eliminate The Food Source For Moles
Moles feed on soil-dwelling insects, especially grubs. You can eliminate this food source by using beneficial nematodes and milky spore to kill the grubs in your soil. The application of milky spore may take several seasons to become effective.;
You can also use a more aggressive grub killer, such as an insecticide. Without food, moles will move on. However, this method is only partially effective. The moles eat earthworms along with other types of worms and insects. They may choose to feast on these instead of leaving your yard.;Carefully follow the directions on any products you use.;
Gophers eat vegetation, so grub killers will not get rid of them.
Recommended Reading: Segment Addition Postulate Find The Length Indicated
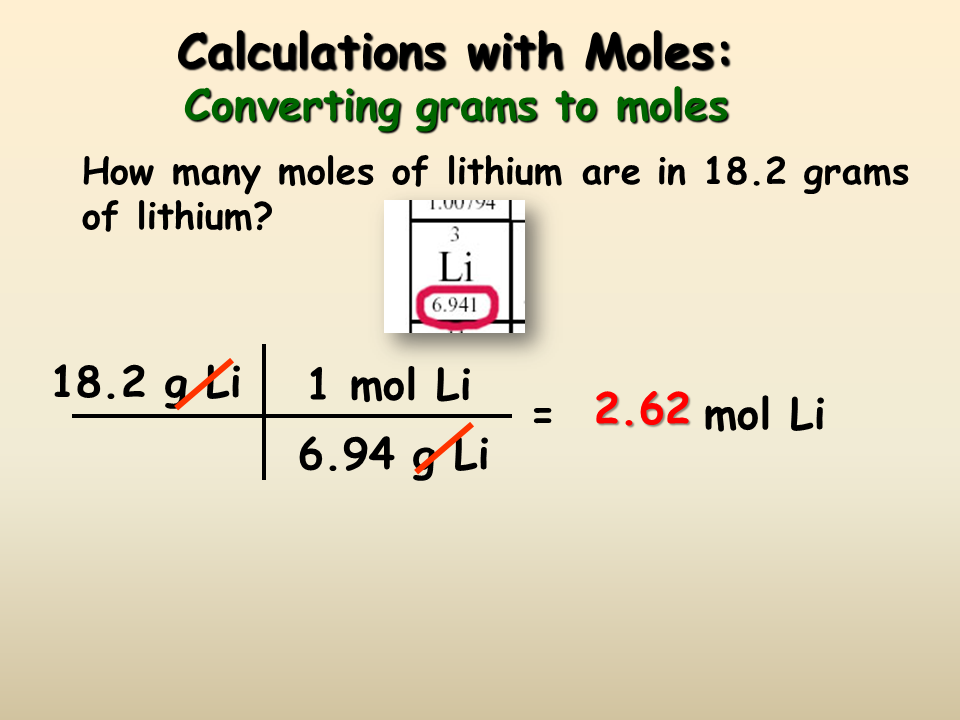
Converting Between Mass Number Of Moles And Number Of Atoms
How many moles and how many atoms are contained in 10.0 g of nickel?
According to the periodic table, the atomic mass of nickel is 58.69 amu, which means that the molar mass of nickel is 58.69 g/mol. Therefore, we can divide 10.0 g of Ni by the molar mass of Ni to find the number of moles present.
Using dimensional analysis, it is possible to determine that:
10\text\times \frac}} = 0.170\text
To determine the number of atoms, convert the moles of Ni to atoms using Avogadros number:
0.170\text\times\frac \text}} = 1.02\times10^\text
Given a samples mass and number of moles in that sample, it is also possible to calculate the samples molecular mass by dividing the mass by the number of moles to calculate g/mol.
What is the molar mass of methane if there are 0.623 moles in a 10.0g sample?
\frac_4}_4} = 16.05 \text_4
The molar mass of CH4 is 16.05 g/mol.
Converting Mass To Number Of Moles
How many moles of NaOH are present in 90 g of NaOH?
Since the molar mass of NaOH is 40 g/mol, we can divide the 90 g of NaOH by the molar mass to find the moles of NaOH. This the same as multiplying by the reciprocal of 40 g/mol.
If the equation is arranged correctly, the mass units cancel out and leave moles as the unit.
90\text\space \text \times \frac}} = 2.25 \space \text
There are 2.25 moles of NaOH in 90g of NaOH.
Read Also: The Segment Addition Postulate Answer Key With Work