Comparison With Surface Observations
Figures and show the annual variation of modeled and measured BrO and IO surface mixing ratios performed at the different Antarctic and Arctic measurements locations compiled in Table of the . Modeled hourly values have been extracted at the closest model grid point with respect to the exact location of each station, and the CAM-Chem output at the surface has been multiplied by the THAMO scaling factor to account for the model variability in the BrO/IO vertical profile within the polar MBL . A 24-hr and a 10-day moving average have been applied to the daytime hourly model output. Equivalent results for the VSLPM-off simulations are shown in Figures and .
Figure 7
| 0.1 | 0.4 |
- aValues for observed and modeled mean, maximum , and standard deviation have been divided by 1013 molec/cm2 for the case of BrO VCDs and by 1012 molec/cm2 for the case of IO VCDs. The model st dev was computed considering the 9:00â11:00 mean value for each day at the closest grid point to each polar base. The satellite st dev considers the monthly variation of all satellite pixels within each model grid point.
- bThe model-observation bias has also been divided by 1013 molec/cm2 for BrO and by 1012 molec/cm2 for IO, while the normalized mean bias and normalized mean error are dimensionless.
- cFor the case of IO VCDs, âCAM-Chem correctedâ values have been used. See text for details.
Templates Based On Halogen Bonds
Halogen bonds have recently been utilized to direct photodimerizations in cocrystals based on templates. Halogen bonds are defined by DXA involve donor and acceptor atoms, with the donor and acceptor sharing a halogen atom.60,61 The halogen atom acts as an electron density acceptor owing to the presence of a region of positive electrostatic potential .62 The group of Metrangolo and Resnati reported the first example of a template based on halogen bonds.63 A tetratopic halogen-bond donor, namely, pentaerythritol tetrakis ether preorganized 4,4-bpe into a face-to-face stacked geometry within infinite 1D ribbons sustained by NI halogen bonds in 2 . In the assembly, the closest C=C bonds, which were disordered, were parallel and separated by less than 4.5 Å. Upon UV irradiation, 4,4-tpcb was generated stereoselectively and in quantitative yield.
Figure 20. Photodimerization of 4,4-bpe using a tetratopic halogen bond template tfipe in 2. Blue-dashed line indicates halogen bonds.
An approach involving halogen bonds was also applied to a photodimerization of vitamin K3 . UV irradiation of pure crystals of vitamin K3 was demonstrated to produce a mixture of syn– and anti-dimers. However, cocrystallization with a ditopic halogen-bond donor, namely 1,4-diiodotetrafluorobenzene , afforded a cocrystal wherein the C=C bonds were parallel and separated by 3.9 Å. UV irradiation of the solid produced pure syn-photodimer of vitamin K3 in near quantitative yield .64
Halogen Elements And Properties
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
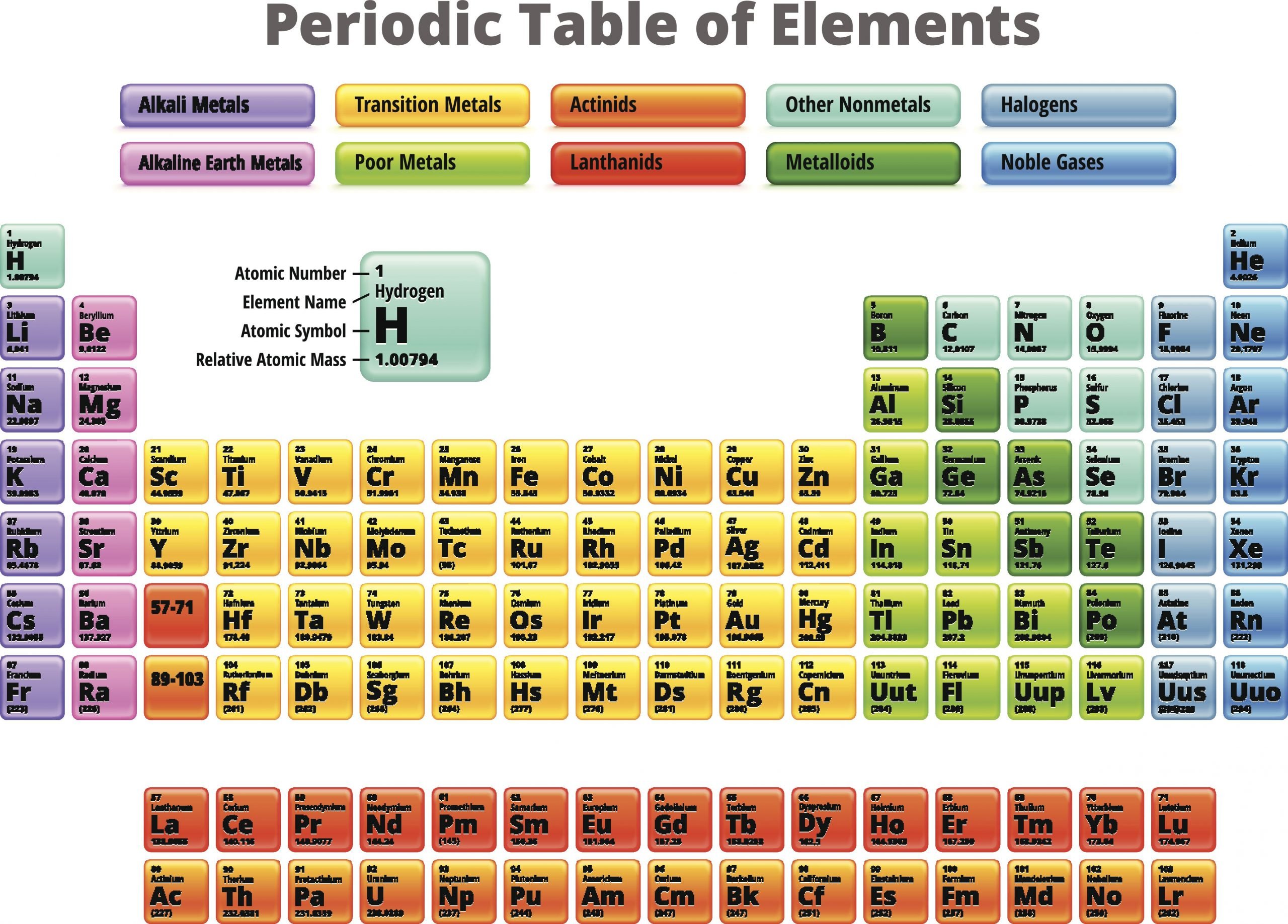
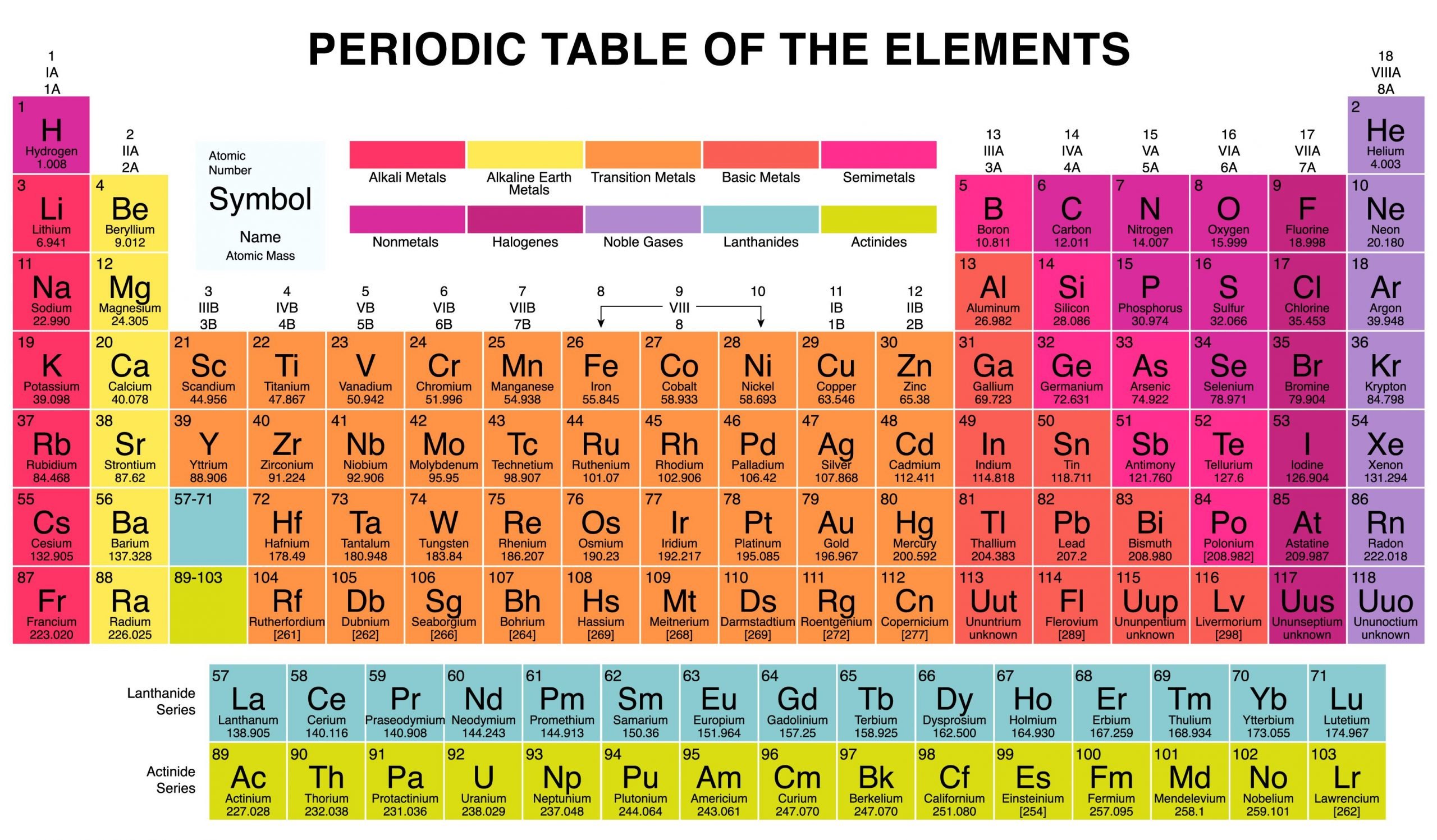
The halogens are a group of elements on the periodic table. It is the only element group that includes elements capable of existing in three of the four main states of matter at room temperature: solid, liquid, and gas.
The word halogen means “salt-producing,” because halogens react with metals to produce many important salts. In fact, halogens are so reactive that they do not occur as free elements in nature. Many, however, are common in combination with other elements Here is a look at the identity of these elements, their location on the periodic table, and their common properties.
Don’t Miss: Chapter 10 Test Form 2a Geometry Answers
Modeling The Sources And Chemistry Of Polar Tropospheric Halogens Using The Cam
Department of Atmospheric Chemistry and Climate, Institute of Physical Chemistry Rocasolano, CSIC, Madrid, Spain
National Research Council , FCEN-UNCuyo, UNT-FRM, Mendoza, Argentina
Department of Chemistry & Environmental Science, Medgar Evers College-City University of New York, Brooklyn, NY, USA
Chemistry Division, Earth and Environmental Science Division, CUNY Graduate Center, Manhattan, NY, USA
Department of Polar Sciences, University of Science and Technology, Incheon, South Korea
Korea Polar Research Institute , Incheon, South Korea
Department of Atmospheric Chemistry and Climate, Institute of Physical Chemistry Rocasolano, CSIC, Madrid, Spain
Correspondence to: A. Saiz-Lopez,
Department of Atmospheric Chemistry and Climate, Institute of Physical Chemistry Rocasolano, CSIC, Madrid, Spain
National Research Council , FCEN-UNCuyo, UNT-FRM, Mendoza, Argentina
Department of Chemistry & Environmental Science, Medgar Evers College-City University of New York, Brooklyn, NY, USA
Chemistry Division, Earth and Environmental Science Division, CUNY Graduate Center, Manhattan, NY, USA
Department of Polar Sciences, University of Science and Technology, Incheon, South Korea
Korea Polar Research Institute , Incheon, South Korea
Department of Atmospheric Chemistry and Climate, Institute of Physical Chemistry Rocasolano, CSIC, Madrid, Spain
Correspondence to: A. Saiz-Lopez,
Model Validation And Performance: Distribution Of Atmospheric Halogens In The Polar Regions
This section presents the validation of the performance of CAM-Chem at reproducing the ambient levels of halogen species in both polar regions when the online polar module is turned on. In doing so, note that the current model configuration was not intended to reproduce a particular set of observations from a specific campaign, but instead to represent the major changes on the background polar halogen abundance when the sea-ice sources are considered.
Recommended Reading: Glencoe Geometry Answers Chapter 7
Effect Of Voltage On Performance
Tungsten halogen lamps behave in a similar manner to other incandescent lamps when run on a different voltage. However the light output is reported as proportional to V . The normal relationship regarding the lifetime is that it is proportional to V 14 } . For example, a bulb operated at 5% higher than its design voltage would produce about 15% more light, and the luminous efficacy would be about 6.5% higher, but would be expected to have only half the rated life.
Halogen lamps are manufactured with enough halogen to match the rate of tungsten evaporation at their design voltage. Increasing the applied voltage increases the rate of evaporation, so at some point, there may be insufficient halogen and the lamp goes black. Over-voltage operation is not generally recommended. With a reduced voltage, the evaporation is lower and there may be too much halogen, which can lead to abnormal failure. At much lower voltages, the bulb temperature may be too low to support the halogen cycle, but by this time the evaporation rate is too low for the bulb to blacken significantly. If the bulbs do blacken, it is recommended to run the lamps at the rated voltage to restart the cycle. There are many situations where halogen lamps are dimmed successfully. However, lamp life may not be extended as much as predicted. The life span on dimming depends on lamp construction, the halogen additive used, and whether dimming is normally expected for this type.
Halides Of The Representative Metals
Thousands of salts of the representative metals have been prepared. The binary halides are an important subclass of salts. A salt is an ionic compound composed of cations and anions, other than hydroxide or oxide ions. In general, it is possible to prepare these salts from the metals or from oxides, hydroxides, or carbonates. We will illustrate the general types of reactions for preparing salts through reactions used to prepare binary halides.
The binary compounds of a metal with the halogens are the halides. Most binary halides are ionic. However, mercury, the elements of group 13 with oxidation states of 3+, tin, and lead form covalent binary halides.
The direct reaction of a metal and a halogen produce the halide of the metal. Examples of these oxidation-reduction reactions include:
Reactions of the alkali metals with elemental halogens are very exothermic and often quite violent. Under controlled conditions, they provide exciting demonstrations for budding students of chemistry. You can view the initial heating of the sodium that removes the coating of sodium hydroxide, sodium peroxide, and residual mineral oil to expose the reactive surface. The reaction with chlorine gas then proceeds very nicely.
The active representative metalsthose that are easier to oxidize than hydrogenreact with gaseous hydrogen halides to produce metal halides and hydrogen. The reaction of zinc with hydrogen fluoride is:
Figure 2.Figure 3.
Don’t Miss: Algebra 1 Chapter 7 Review Answer Key
Quantification Of Halogen Emissions From The Polar Regions
This section quantifies the sea-ice halogen emissions and compares them to the ocean VSL halocarbon fluxes already implemented in the benchmark CAM-Chem setup within each polar cap . We have also conducted sensitivity runs to estimate the uncertainty in the sea-ice emission flux by iteratively modifying the flux from our best-adjusted value until the bias shows a persistent disagreement with observations. We estimate an uncertainty of ±50% in the computed halogen emission flux. Beyond this uncertainty range, the model either clearly underestimates or overestimates the observations. To help with readability, we ordered the text so that results in sections to correspond to processes described in sections to , respectively. Finally, section compares the contribution of each independent source and provides individual mean results for different seasons. All the annual flux estimations presented here have been performed for the VSLPM-on experiment unless stated otherwise.
Halogen Bonding In Biological Macromolecules
For some time, the significance of halogen bonding to biological macromolecular structure was overlooked. Based on single-crystal structures in the protein data bank , a study by Auffinger and others on single crystals structures with 3 Ã resolution or better entered into the PDB revealed that over 100 halogen bonds were found in six halogenated-based nucleic acid structures and sixty-six protein-substrate complexes for halogen-oxygen interactions. Although not as frequent as halogen-oxygen interactions, halogen-nitrogen and halogen-sulfur contacts were identified as well. These scientific findings provide a unique basis for elucidating the role of halogen bonding in biological systems.
For molecular recognition and binding, halogen bonding can be significant. An example of this assertion in drug design is the substrate specificity for the binding of IDD 594 to human aldose reductase. E.I. Howard reported the best resolution for this monomeric enzyme. This biological macromolecule consists of 316 residues, and it reduces aldoses, corticosteroids, and aldehydes. D-sorbitol, a product of the enzymatic conversion of D-glucose, is thought to contribute to the downstream effects of the pathology of diabetes. Hence, inhibiting this enzyme has therapeutic merit.
Also Check: Prentice Hall Gold Geometry Answer Key
Health Safety And Technical Notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Take care to limit students exposure to chlorine and bromine water fumes. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed.
- Chlorine water, Cl2 see CLEAPSS Hazcard HC022b and CLEAPSS Recipe Book RB025. The solution itself is LOW HAZARD but chlorine gas escapes, so a HARMFUL label would be sensible.
- Bromine water, Br2 , see CLEAPSS Hazcard HC015b and CLEAPSS Recipe Book RB017.
- Iodine solution, I2 see CLEAPSS Hazcard HC054 and CLEAPSS Recipe Book RB050. Iodine solution is actually iodine dissolved in aqueous potassium iodide.
- Potassium chloride, KCl, potassium bromide, KBr and potassium iodide, KI solutions are all LOW HAZARD see CLEAPSS Hazcard HC047b and CLEAPSS Recipe Book RB068. The sodium salts can be used if the potassium salts are not available. The concentration of the potassium iodide solution should be adjusted so that it gives a light brown solution on addition of chlorine water. If the reagents are too concentrated, a black precipitate of iodine often results instead of a brown solution.
- Cyclohexane, C6 H12 , see CLEAPSS Hazcard HC045b.
Formation Of Ionic Compound
Halogens are very reactive non-metals, they react with metals to form ionic salts. For example, sodium burns in chlorine to form sodium chloride. A bright flame is observed in this reaction.
Halogens form an ion with a charge of -1. Each halogen atom has seven electrons in the valence shell. One electron is taken in to achieve stable noble gas octet electronic configuration. The ions of halogens are called halides. Ion of fluorine is fluoride, ion of chlorine is chloride, ion of bromine is bromide and that of iodine is iodide.
The compounds that halogens formed with metals are all ionic.
The halogens become less reactive down the Group. Fluorine is the most reactive non-metal in the Periodic Table. Chlorine is more reactive than bromine, and bromine is more reactive than iodine.
Read Also: Holt Geometry Practice Workbook Answer Key Pdf
What Are Halogen Elements
The halogen elements are the six elements in Group 17 of the periodic table. Group 17 occupies the second column from the right in the periodic table and contains fluorine , chlorine , bromine , iodine , astatine , and tennessine . Astatine and tennessine are radioactive elements with very short half-lives and thus do not occur naturally.
Chemistry End Of Chapter Exercises
reaction of a weak base and a strong acid
\text_3\ +\ \text_4\
preparation of a soluble silver salt for silver plating
\text_2\text_3\ +\ \text_3\
preparation of strontium hydroxide by electrolysis of a solution of strontium chloride
\text_2\ +\ \text_2\text\ \xrightarrow}
\text_3^
PCl5
SeF4
ClF3
BrF3
NaClO4
KClO
H5IO6
\text_4^
ClO2
ICl3
Don’t Miss: Which Founding Contributors To Psychology Helped Develop Behaviorism
Examples Of Pseudohalogen Molecules
Examples of symmetrical pseudohalogens include cyanogen 2, thiocyanogen 2, hydrogen peroxide H2O2. Another complex symmetrical pseudohalogen is dicobalt octacarbonyl, Co28. This substance can be considered as a dimer of the hypothetical cobalt tetracarbonyl, Co4.
Examples of non-symmetrical pseudohalogens , analogous to the binary interhalogen compounds, are cyanogen halides like ClCN or BrCN nitryl fluoride, and other compounds. Sometimes nitrosyl chloride NOCl also is considered as pseudohalogen.
Not all combinations are known to be stable.
Halogens In Aqueous Solution And Their Displacement Reactions
In association with Nuffield Foundation
- Five out of five
This class experiment or demonstration explores some of the chemical properties of halogens, comparing the colours of three halogens in aqueous solution and in a non-polar solvent, and observing their bleaching properties and displacement reactions
Halogens react to a small extent with water, forming acidic solutions with bleaching properties. They also undergo redox reactions with metal halides in solution, displacing less reactive halogens from their compounds. These displacement reactions are used to establish an order of reactivity down Group 17 of the periodic table.
This series of simple experiments illustrates some of the chemical properties of the halogens following an introduction to the physical properties of the Group 17 elements. It can be done as a demonstration or as a class experiment.
Investigating the solubility of the halogens in a non-polar solvent can be left out, or only shown as a demonstration.
If the activity is done as a demonstration it should take around 15 minutes. If it is done as a class experiment you should allow 30 minutes.
Recommended Reading: Which Of The Following Perspectives Dominated American Psychology For Decades
Electronic Configuration Of Group 17 Elements
The valence shell electronic configuration of these electrons is ns2np5. Thus, there are 7 electrons in the outermost shell of these elements. The element misses out on the octet configuration by one electron. Thus, these elements look out to either lose one electron and form a covalent bond or gain one electron and form an ionic bond. Therefore, these are very reactive non-metals.
To The Carbon Atom That Is Bonded To The Halogen
Read another article:How much does it cost to take the metro in montrealHow much does gamestop pay for used xbox oneHow much does the metro cost in madridHow much does the master chief collection costHow much is a covid test without insurance at urgent care
Addition of halogens to an Organic compound. In the Greek language halo means salt and gen means formation which gives the meaning of salt formation. However d-block elements within a period are more similar to each other. The halogens are all highly reactive which means theyre quick to form bonds with other elements. The Group 7 elements are called the halogens.
Halogenation is a chemical reaction involving the addition of one or more halogens to a substance or product. The term halogen originates from a combination of Greek words meaning salt-producing. Fluorine gas is deadly. The elements in group 7 are called the halogens. Alkyl Halides And Elimination Reactions Organic Reactions Reactions Functional Group.
Addition of halogens to an Organic compound. The elements of halogens are fluorine Chlorine Bromine Iodine and Astatine. Halogen any of the six nonmetallic elements that constitute Group 17 Group VIIa of the periodic table. We will look at some of the physical and chemical properties of Halogens. .
Also Check: Algebra 2 Chapter 4 Test Form A
Fun Fact About The Halogens
- Many rare-earth fluorides are insoluble in water
- Students learn that silver chloride is insoluble, but most people dont know that copper chloride is also insoluble in water
- Bromide and bromate ions are key ions in a famous and beautiful oscillating reaction known as the BelousovZhabotinsky reaction
- Iodate ions play a key role in many clock reactions used for chemical demonstrations
- Astatine is the rarest element that occurs naturally in the earths crust. A sample of pure astatine has never been assembled. All isotopes are radioactive and short-lived.
- Although hydrofluoric acid, HF, is a weak acid, it is one of the most dangerous acids to be around. It not only damages the body on the outside but it can be absorbed through the skin, penetrating deep into tissues causing severe systemic injury
- Chlorine gas killed thousands of soldiers during world war I in 1915, when used as a chemical weapon. Ok that is not a very fun fact.
- Iodine will sublime directly from a solid into a gas. It will also enter the liquid state at 114C if heated carefully in a vessel that is not too open
- The hypochlorite ion, ClO, is the active ingredient in bleach.