Mass Percentage: Meaning Formula Examples
Content Curator| Updated On -May 1, 2022
Mass percentage is a way of expressing the concentration or component of a particular mixture. The composition of a solution can be expressed in mass percentage, which indicates the mass of the solute in the mass of a given solution. The amount of solute is expressed in mass or mol. For solutions, multiply the number of grams of solute with the gram of solution by 100 to determine the mass percentage.
Key Terms: Mass percentage formula, molar mass, percentage of compound.
Find The Percent Abundance
To get percent abundance, we will multiply the relative abundance value by 100 and put a percent sign.
As we have now x= 0.76. Simply, multiply by 100 to get percent. So, chlorine-35 is 0.76*100=76%
Now,
= = 0.24
Multiplying above by 100, we get 24%.
Therefore, the percent abundance of chlorine-35 is 76% and the percent abundance of chlorine-37 is 24%.
Density: A Further Investigation
We know all of density’s components, so let’s take a closer look at density itself. The unit most widely used to express density is g/cm3 or g/mL, though the SI unit for density is technically kg/m3. Grams per centimeter cubed is equivalent to grams per milliliter . To solve for density, simply follow the equation d = m/v. For example, if you had a metal cube with mass 7.0 g and volume 5.0 cm3, the density would be
Sometimes, you have to convert units to get the correct units for density, such as mg to g or in3 to cm3.
Density can be used to help identify an unknown element. Of course, you have to know the density of an element with respect to other elements. Below is a table listing the density of a few elements from the Periodic Table at standard conditions for temperature and pressure, or STP corresponding to a temperature of 273 K and 1 atmosphere of pressure.
| Element Name and Symbol | |
|---|---|
| 22.6 | 76 |
As can be seen from the table, the most dense element is Osmium with a density of 22.6 g/cm3. The least dense element is Hydrogen with a density of 0.09 g/cm3.
You May Like: Is Pre Algebra Advanced For 7th Grade
How To Calculate Percentage Of A Number
It is possible to calculate the percentage in three simple steps. They are:
Step 1: Identify the original form of the number, i.e. fraction or decimal. The original format will define the following mathematical operation on the number. Suppose a decimal number is 0.25, which may be the calculated ratio of the values we are comparing, while an example of a fraction is 4/15.
Step 2: Perform the mathematical operation on the number. That means, if the given number is a fraction, convert it to a decimal number. If the given number is a decimal number, then leave it as it is.
For example, 4/15 = 0.267
Step 3: Multiply the result obtained in the previous step by 100 and the resulted value should be expressed as a percentage. For example, 0.267 × 100 = 26.7%.
Learn how to convert decimal to fraction here.
Alternative Method:
We can also calculate the percentage of a number by changing the number into a fraction with the denominator 100. This can be understood in a better way from the example given below:
Example:
Convert 3/20 to a percentage.
Solution:
The given fraction is 3/20.
Let us convert the denominator to 100.
× = 15/100
Now, multiply the result by 100.
× 100 = 15
Therefore, 3/20 = 15%
Note: This method of calculating the percentage is applicable only when the denominator is a factor of 100. Otherwise, we can use the regular method to calculate the percentage.
Elements Pulling Their Weight
There is another way to look at the composition of sodium chloride. Sodium, being the lighter element, takes up 39% of the total mass of the compound. Chlorine, the heavyweight, takes up the remaining 61% of the total mass. We call this the percentage mass of an element in a compound.
How did we get these percentages? We express the mass of all the atoms of the element we are interested in as a fraction of the total mass of the compound:
% mass of element Z = ÷ Mr of compound × 100%
Don’t Miss: What Is An Equation In Chemistry
What Is The Percent Mass Calculator
This calculator is a very useful tool that helps you to calculate the overall ratio of the mass component in a substance with the total mass of the substance.
So, here we represent the percentage %. keep in mind that you need to add the mass of solute and solvent of the desired solution, it is good for you if you put values in grams.
After completing this step, the percent mass calculator gives you the percentage of your mass solute in a chemical compound solution. Also, this depends on the mass percent formula which you are using.
The other amazing thing is that you can also calculate the percent composition of the desired mixture. All you need to do is just enter the number of atoms of the desired element, which are present in the mixture.
Tip:
Always remember that the mass percentage of the desired solution is independent of the temperature.
How To Calculate Percentage Of Marks
Most of the students get a question on how to calculate the percentage of marks obtained in exams. Here is the answer to this question along with the example. Two simple steps give you the percentage of marks. They are:
Step 1: Divide the obtained marks by the maxim marks of the test.
Step 2: Multiply the result by 100.
Go through the example given below to understand the process of finding the percentage of marks.
Example 1: A student scored 1156 marks in the examination out of 1200 marks. Calculate the percentage of marks secured by the student.Go through the example given below to understand the process of finding the percentage of marks.
Solution:
Number of marks scored = 1156
Maximum marks = 1200
Percentage of marks = × 100
Percentage= 0.9633. × 100
Therefore, the percentage of marks obtained is 96.3%
Also Check: Kuta Software Algebra 1 Two Step Equations
S For Finding The Mass Percentage
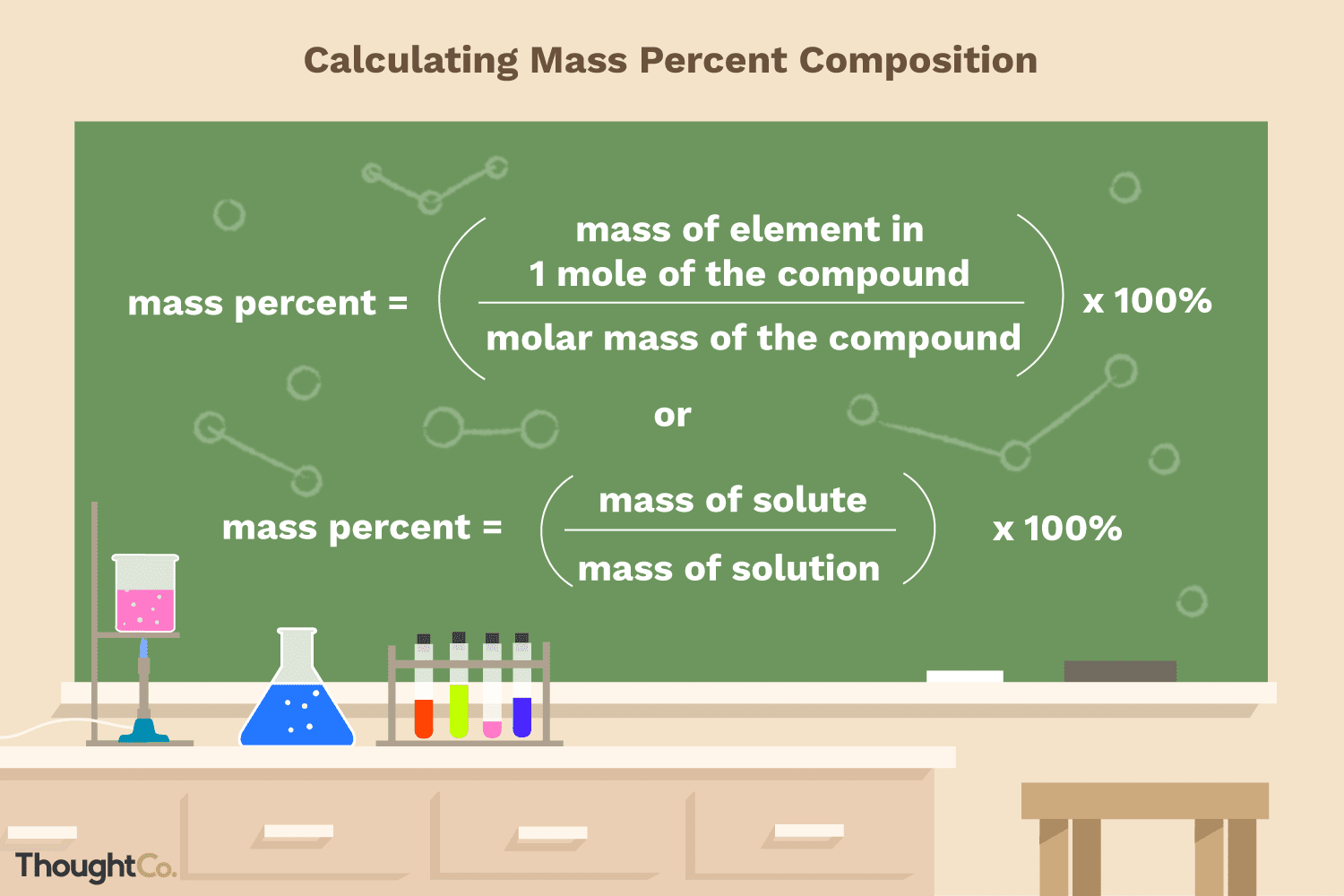
- Step 1: Define the equation for mass percent of a compound. The essential formula for mass percent of a compound is mass percent = x 100. You want to multiply by 100 at the top to specify the value as a percentage.
- Step 2: Calculate the entire mass of the compound. once you know the masses of all the elements or compounds being added together, you merely got to add them together to calculate the entire mass of the ultimate compound or solution. And this may be the denominator within the mass percent calculation.
- Step 3: Identify the mass of the chemical-in-question. When asked to seek out the mass percent, youre being asked to seek out the mass of a specific chemical as a percentage of the entire mass of all elements. Then write down the mass of the chemical-in-question. This mass is the numerator within the mass percent calculation.
- Step 4: The variables into the mass percent equation. So once youve got determined the values for every variable, plug them into the equation.
- Step 5: Calculate the mass percent. The equation is filled in, so now simply solve to calculate the mass percent. Divide the mass of the chemical by the entire mass of the compound and multiply by 100. This may offer you the mass percent of the chemical.
Tips For Success Calculating Mass Percent
- You won’t always be given the total mass of a mixture or solution. Often, you’ll need to add up the masses. This might not be obvious. You may be given mole fractions or moles and then need to convert to a mass unit.
- Watch your significant figures.
- Always make sure the sum of the mass percentages of all components adds up to 100%. If it doesn’t, you need to go back and find your mistake.
Recommended Reading: What Does N Stand For In Physics
Discover And Learn What Students Are Asking
?In Exercises 1-8, verify the solution of the differential equation.Solution D?The following diagrams represent two electromagnetic waves, drawn on the same scale. Which wave has a longer wavelength? W?Priming for Healthy Food Does alerting shoppers at a grocery store regarding the healthiness of energy-dense snack foods change the ?Weight Gain and Gender According to the National Vital Statistics Report, 20.1% of all pregnancies result in weight gain in excess of 40 pounds . Is it rea?Elapsed Time to Earn a Bachelors Degree A researcher with the Department of Education followed a cohort of students who graduated from high school in?Given the following ANOVA output, answer the questions that follow Is there evidence of an interaction effect? Why or why not??Consider the following equation:\+\mathrm^ \longrightarrow \mathrm\)Which of the following stateme
Is There Any Difference Between Mass Percent And Percent Composition
Answer. The concept of mass percent and percent composition are related to each other. But the main difference is that mass percent gives you the percentage ratio of the component in a mixture, with the total mass of the substance. On the other hand, the percent composition is that it will represent the above percentage, but it is the mass of each element that you use in the mixture.
Don’t Miss: The Algebra Of Happiness Scott Galloway
Calculate The Change In Mass
Once you have the initial and final masses of your substance, subtract to determine the difference. This simple calculation shows the amount the mass has changed. The smaller of the two masses is always subtracted from the larger, regardless of which is initial or final. For the water experiment, you’d subtract the smaller final mass from the larger initial mass:
You can see from this calculation that the mass of the water changed by 0.15 kilograms over the course of your experiment.
What Is Mass Percent Mass Percent And Percent Composition
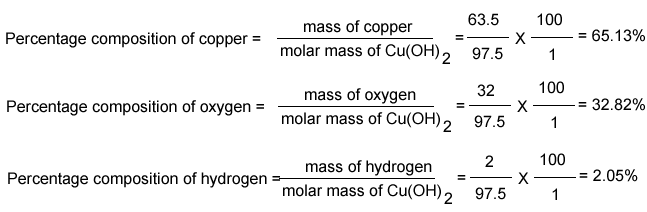
Now that you know how to find the mass percent of a solution with our calculator, it’s time to learn about mass percent definition:
The mass percentage represents the concentration of a component of a mixture or compound. For solutions, the mass percent represents the ratio of solute to the total solution, as a percentage.
The mass percent and percent composition are two distinct concepts that are usually confused, as they both represent the percentages of components. The main difference between mass percent and percent composition is:
- The mass percent gives the percentage ratio of the mass of a component in a mixture and the total mass of the substance and
- The percent composition represents the above percentages but as the mass of each element in the mixture.
Recommended Reading: How To Study Physics Chemistry And Biology
How To Calculate Percentage Change
Lets learn how to calculate the percentage difference between two numbers. The percentage difference is the variation in the value of a number or quantity over a while in terms of percentage.
The formula gives Percentage change is:
% change = × 100
Here,
Change in Value = New value Original value
The change in the value could be positive or negative. Positive difference means there is a percentage increase in the value, otherwise, it is called percentage decrease.
Thus, there are two types of percentage change in mathematics. They are:
- Percentage increase
- Percentage decrease
Mass Percentage Is A Way Of Expressing The Concentration Of Solution
There are several ways of expressing the concentration of a solution by using a percentage. The mass percentage is one of the ways to express the concentration of a dissolved substance in a solution.
The mass percentage refers to the ratio of a mass of a compound in the solution to the total mass of the solution.
For instance, calculate the mass percent concentration for the solution obtained by dissolving 10 g of sodium chloride and 6g of sodium bicarbonate in 120g of water.
Add up the mass of all compounds in the solution, including the solvent, to calculate the total mass of the solution.
Also Check: What Is An Exponent In Algebra
How To Calculate Percentage Using Formula
We can use the formula to calculate the percentage easily and quickly. The formula to calculate percentage is equal to the ratio of the actual value to the total value multiplied by 100. The percentage formula is:
| \ |
Let us understand this concept and formula with the help of an illustrative example given below:
Example: A circle below has ten divisions, and a few parts are shaded. Calculate the percentage of shaded parts in the circle.
Solution:
Total number of divisions in the circle = 10
Number of shaded parts = 2
We know that,
Percentage, % = × 100
Thus, the percentage of the shaded part of the circle = × 100
= × 100
If The Percentage Yield Of This Reaction Is 80% Calculate The Mass Of Magnesium That Needs To Be Burned To Produce 30g Of Magnesium Oxide
Percentage yield is defined as actual yield / theoretical yield .
We know the actual yield is 30 g of magnesium oxide , and we know the yield is 80% . So using this information, AND THE CORRECTLY BALANCED EQUATION, we can find the mass of Mg needed.
Burning Mg:
2Mg + O2 ==> 2MgO … balanced equation
molar mass MgO = 40.30 g/mol
30 g MgO x 1 mol / 40.30 g = 0.744 mols MgO
From the balanced equation we can convert this to mols of Mg needed:
0.744 mols MgO x 2 mols Mg / 2 mols MgO = 0.744 mols Mg needed, if the yield were 100%
Since yield is only 80%, we need 20% more to account for this deficiency:
Finally, we convert this amount of Mg to grams:
0.893 mols Mg x 24.3 g Mg/mol = 21.7 g Mg = 20 g Mg
You May Like: What Is Nonlinear In Math
Percent Mass Calculator In Chemistry Volume Change Etc
If you have difficulty finding the ratio between the mass component in a substance and the total mass of the substance, then the Percent Mass Calculator will help you in this matter.
Lots of people are also confused about the difference between percent composition and mass percent. So, in this review, you will get all the information, which gives you a better understanding of the percent mass calculator. Are you ready to dive into depth details, Lets start!
Percent Composition In Everyday Life
Percent composition plays an important role in everyday life. It is more than just the amount of chlorine in your swimming pool because it concerns everything from the money in your pocket to your health and how you live. The next two sections describe percent composition as it relates to you.
Nutrition Labels
The nutrition label found on the container of every bit of processed food sold by the local grocery store employs the idea of percent composition. On all nutrition labels, a known serving size is broken down in five categories: Total Fat, Cholesterol, Sodium, Total Carbohydrate, and Protein. These categories are broken down into further subcategories, including Saturated Fat and Dietary Fiber. The mass for each category, except Protein, is then converted to percent of Daily Value. Only two subcategories, Saturated Fat and Dietary Fiber are converted to percent of Daily Value. The Daily Value is based on a the mass of each category recommended per day per person for a 2000 calorie diet. The mass of protein is not converted to percent because their is no recommended daily value for protein. Following is a picture outlining these ideas.
Penny: The Lucky Copper Coin
Today, the penny in America is 2.5% copper with 97.5% zinc. The copper coats the outside of the penny while the inner portion is zinc. For comparison’s sake, the penny in Canada is 94% steel, 1.5% nickel, and 4.5% copper.
Don’t Miss: August 15 Geometry Regents Answers
What Is The Mathematical Formula For Calculating Mass Percent Composition From A Chemical Formula
Find the molar mass, Find the mass of each element Divide the mass of the element by the molar mass x 100 = %
Explanation:
The percent composition is the sum of all the percentages of each element in the compound.
The molecular formula mass is 100% or the total of the compound. So the first thing is to find the molar mass from the chemical formula.
Then find the mass of each of each element in the compound. For example ethanol has the formula # C_2H_5OH # To find the mass of each element multiply the atomic mass by the number of atoms.
6 x 1 = 6 grams of Hydrogen 2 x 12 = 24 grams of Carbon. 1 x 16 = 16 grams of Oxygen Total = 45 grams total = the formula mass of the compound.
The divide the mass of each element by the molecular formula mass
# 6/ 46#
What Is The Equation For The Percentage Mass Of An Element In A Compound
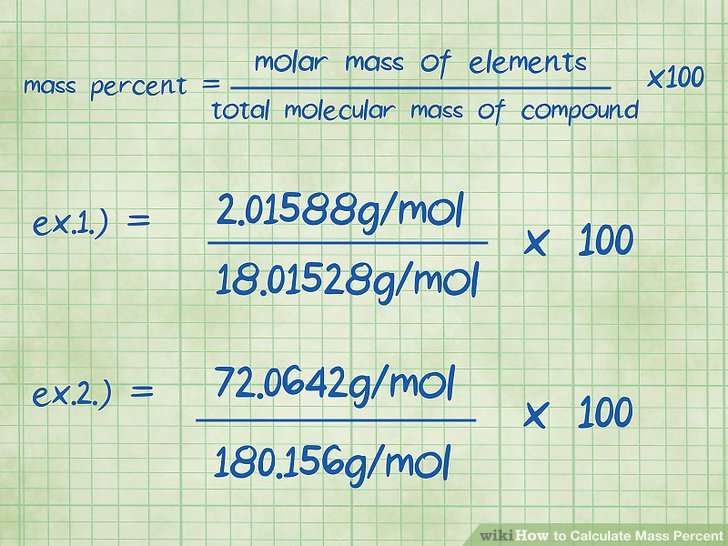
1 year ago
Verified Sherpa Tutor
% mass = x 100
Make sure you consider the subscript numbers in the chemical formula. Check out https://www.bbc.co.uk/bitesize/guides/zs3297h/revision/4 for worked examples.
Experienced GCSE Science and A level Chemistry tutor with Phd Interested in booking a 1-1 lesson with me?
Got the answer? Help Stacey out.
Don’t Miss: Pearson Texas Geometry Form G Answers
How To Calculate Mass Percent
This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, several readers have written to tell us that this article was helpful to them, earning it our reader-approved status. This article has been viewed 584,317 times.
Mass percent tells you the percentage of each element that makes up a chemical compound.XResearch source Finding the mass percent requires the molar mass of the elements in the compound in grams/mole or the number of grams used to make a solution.XResearch source It is simply calculated using a basic formula dividing the mass of the element by the mass of the compound .