Value Of Ideal Gas Constant In Si Unit
At STP , the molar volume or volume per mole is 22.414 × 103 m3 mol1. Therefore, we can calculate the value of R as
This is an approximate value of the ideal gas constant.
With the 26th General Conference on Weights and Measures , the revised and exact value of the gas constant is 8.314 462 618 153 24 J mol1 K1.
Ideal Gas Equation And Nernst Equation
The ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature:PV = nRTHere, P is pressure, V is volume, n is number of moles of an ideal gas, R is the gas constant, and T is temperature.
The Nernst equation relates the reduction potential of a half-cell to the standard electrode potential, temperature, moles of electrons, Faradays constant, and reaction quotient:E = E0 lnQHere, E is the cell potential, E0 is the standard cell potential, R is the gas constant, T is the temperature, n is the number of mole of electrons exchanged, F is Faradays constant, and Q is the reaction quotient.
Notation For The Gas Constant
The gas constant defined in this article is the universal gas constant, R, that applies to any gas. There is also a specific gas constant, which can be denoted as Rs. The specific gas constant is defined as Rs = R / M where M is the molecular weight.
Unfortunately, many authors in the technical literature sometimes use R as the specific gas constant without denoting it as such or stating that it is the specific gas constant. This can and does lead to confusion for many readers.
Read Also: Eoc Fsa Warm Ups Algebra 1 Answers
Rydberg Unit Of Energy
- 1 J }\equiv hcR_=}e^}^h^}}=2.179\ 872\ 361\ 1035\times 10^\ }}
- }}=9.112\ 670\ 505\ 824\times 10^\ }} .
- m }}=1.450\ 326\ 555\ 7696\times 10^\ }} .
The Bohr model explains the atomic spectrum of hydrogen as well as various other atoms and ions. It is not perfectly accurate, but is a remarkably good approximation in many cases, and historically played an important role in the development of quantum mechanics. The Bohr model posits that electrons revolve around the atomic nucleus in a manner analogous to planets revolving around the sun.
In the simplest version of the Bohr model, the mass of the atomic nucleus is considered to be infinite compared to the mass of the electron, so that the center of mass of the system, the barycenter, lies at the center of the nucleus. This infinite mass approximation is what is alluded to with the subscript. The Bohr model then predicts that the wavelengths of hydrogen atomic transitions are :
- 1 2 ) }=\mathrm \cdot \left=}e^}^h^c}}\left}
where n1 and n2 are any two different positive integers , and is the wavelength of the emitted or absorbed light.
- 1 2 ) }=R_\left}
where , =R_/,} and M is the total mass of the nucleus. This formula comes from substituting the reduced mass of the electron.
The Rydberg constant can also be expressed as in the following equations.
- R a 0 =m_}c}}=}}}}=}}}
- 0 . =}m_}c^\alpha ^=}m_}})^\hbar ^}}=}}c^r_}}}}=}}}}}=}hf_}\alpha ^=}\hbar \omega _}\alpha ^=}}}\left^=}})a_}}.}
where
Limitations Of Ideal Gas Law
There is a reason it is called the ideal gas law instead of the actual gas law. The validity of the ideal gas equation depends on a handful of idealized assumptions about the character and behavior of gases. First, the ideal gas law assumes that particles in a gas obey Newtons laws of mechanics. This means that gas particles are assumed to obey the laws of force and gravity described by Isaac Newton and the effects of electrostatic intermolecular attractions are not considered.
Todays science fiction is tomorrows science fact. Isaac Asimov
Second, it is assumed that the molecules of the gas are negligibly small compared to the entire volume of the gas. This assumption allows scientists to simplify their calculations for the volume by leaving out the non-zero volume that molecules actually have.
Thirds, collisions between the molecules and the walls of the container are considered to be perfectly elasticthat is, no kinetic energy is lost from collisions. In actuality, a tiny amount of kinetic energy is absorbed by the walls of the container and is dissipated as heat. Normally, this tiny amount of energy is negligible and can be ignored.
Read Also: Difference Between Electron Geometry And Molecular Geometry
What Is The Value Of Rh
value
Hereof, what is Rh equal to?
RH = 2.179 x 10-18 Joules n is the Principal Quantum Number.
Likewise, what is n1 and n2 in Rydberg equation? n1 and n2 are integers and n2 is always greater than n1. R is constant, called Rydberg constant and formula is usually written as. The modern value of Rydberg constant is 109677.57 cm-1 and the most precise known physical constant. Example 1. Determine the ¯ for the transition wherein n1=6 to n2 = 3 in a hydrogen atom
Correspondingly, what is Rh chemistry?
In spectroscopy, the Rydberg constant, symbol for heavy atoms or for hydrogen, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to the electromagnetic spectra of an atom.
What is the Rydberg formula used for?
The Rydberg formula is a mathematical formula used to predict the wavelength of light resulting from an electron moving between energy levels of an atom. When an electron changes from one atomic orbital to another, the electron’s energy changes.
In Pv=nrt What Is The R Constant
In chemistry, the formula PV=nRT is the state equation for a hypothetical ideal gas. The ideal gas law describes the behavior of an ideal sample of gas, and how that behavior is related to the pressure , temperature , volume , and molarity of the gas sample. In the equation PV=nRT, the term R stands for the universal gas constant.
The universal gas constant is a constant of proportionality that relates the energy of a sample of gas to the temperature and molarity of the gas. It is sometimes called the ideal gas constant, the molar gas constant. It is also sometimes called the Regnault constant, in honor of the French chemist Henri Regnault whose quantitative data was first used to precisely calculate the value of the constant. The currently accepted value for the universal gas constant R is:
R Constant = 8.3144598 J/mol·K
The unit for the gas constant is the joule per mol-kelvin. This can be read as work per mol per degree Essentially, the gas constant relates the molar amount of gas and temperature of the gas to the amount of kinetic energy in the gas. One can calculate the universal gas constant by dividing the product of the pressure and volume of a gas by the molarity and temperature of the gas:
R = PV/nT
Read Also: Geometry Dash Demon Key Hack
What Does The Constant R In The Ideal Gas Law Mean
Regnault constant, or Universal Gas Constant.
Explanation:
Gas constant, R, is named after the French chemist Henri Victor Regnault. It is also called the Universal Gas Constant.
Gas constant is equivalent to Boltzmann constant #k_B# , expressed in terms of energy.
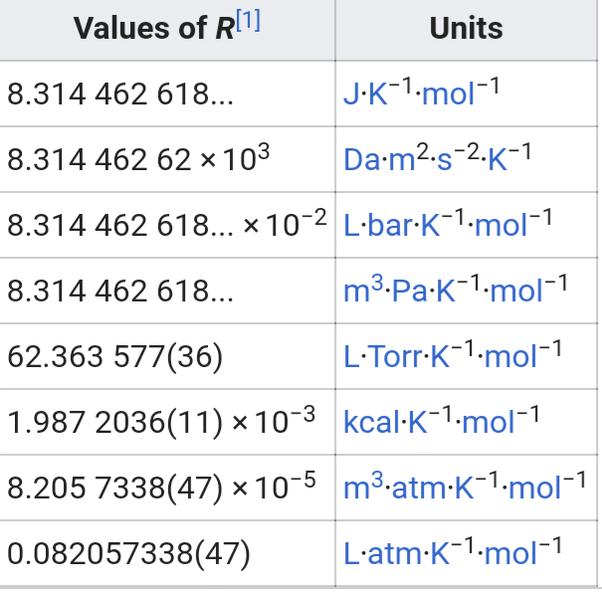
There are many values of R depending on the units used.
Here are some examples:
#”amu” cdot/”s”^2″K”^# #8.314# #”m”^3″bar”cdot”K”^”mol”^# #8.314xx10^# #”L””bar”cdot”K”^”mol”^”# #62.36#
R can be viewed as a scaling factor for molar energy of ideal gas law. For ideal gas law, energy can be viewed as increasing linearly with temperature. #E=alphaT#
Through derivation. it was shown that for one-dimension, #E=1/2 RT# while for 3 dimension #E=3/2 RT# The above is for 1 mole of ideal gas. For 1 molecule of gas, we know that #R=N_ k# So 1 molecule of ideal gas moving in 3 dimension is #E=3/2 kT# You can equate the kinetic energy of the molecule #E=1/2 m v^2# to previous equation and get #E=3/2 kT= 1/2 m v^2#
Read more
What Is The R Constant In Gibbs Free Energy
Rgas constantfree energyfree energy
. Similarly, it is asked, what is r in Gibbs free energy?
Free energy and Equilibrium ConstantsG = standard-state free energy. R = ideal gas constant = 8.314 J/mol-K. T = temperature
Similarly, what is the difference between Gibbs free energy and standard free energy? Free energy or Gibbs function is by definition g = h – Ts, where h is enthalpy , T is absolute temperature and s is entropy enthalpy is defined as that based on a reference wherein the value is zero for the elemental substances.
Additionally, what is the relation between Gibbs free energy and equilibrium constant?
A non-spontaneous reaction has a positive delta G and a small K value. When delta G is equal to zero and K is around one, the reaction is at equilibrium. You have learned the relationship linking these two properties. This relationship allows us to relate the standard free energy change to the equilibrium constant.
What do you mean by free energy explain?
In physics and physical chemistry, free energy refers to the amount of internal energy of a thermodynamic system that is available to perform work. Landau free energy describes energy of an open system in which particles and energy may be exchanged with the surroundings.
Don’t Miss: Does Mj Have Any Biological Kids
Deriving The Ideal Gas Law
Now that we have the 4 fundamental state equations for gas, we can combine them into one single expression to yield the ideal gas law. We can combine laws like this:
- V T
- V 1/P
- P T
- V n
Combining these expressions gives us:
- V nT/P
Since represent direct proportionality, we can replace the with a = by adding a constant of proportionality to the right-hand side. Experimentally, we have verified this constant to be equal to the value of R, so adding R to the equation yields::
- V = nRT/P
Rearranging this equation gives us:
- PV = nRT
Measurement And Replacement With Defined Value
As of 2006, the most precise measurement of R had been obtained by measuring the speed of sound ca in argon at the temperature T of the triple point of water at different pressures P, and extrapolating to the zero-pressure limit ca. The value of R is then obtained from the relation
- c u , }=RT} }M_ }}}},}
where:
- 0 is the heat capacity ratio
- T is the temperature, TTPW = 273.16 K by the definition of the kelvin at that time
- Ar is the relative atomic mass of argon and Mu = 103 kgmol1 as defined at the time.
However, following the 2019 redefinition of the SI base units, R now has an exact value defined in terms of other exactly defined physical constants.
Recommended Reading: Lesson 4.5 Practice B Geometry Answers
Relationship With The Boltzmann Constant
The Boltzmann constantkB may be used in place of the molar gas constant by working in pure particle count, N, rather than amount of substance, n, since
- R , }k_},\,}
where NA is the Avogadro constant.For example, the ideal gas law in terms of the Boltzmann constant is
- P , }T,}
where N is the number of particles , or to generalize to an inhomogeneous system the local form holds:
- P
where N is the number density.
The Ideal Gas Constant
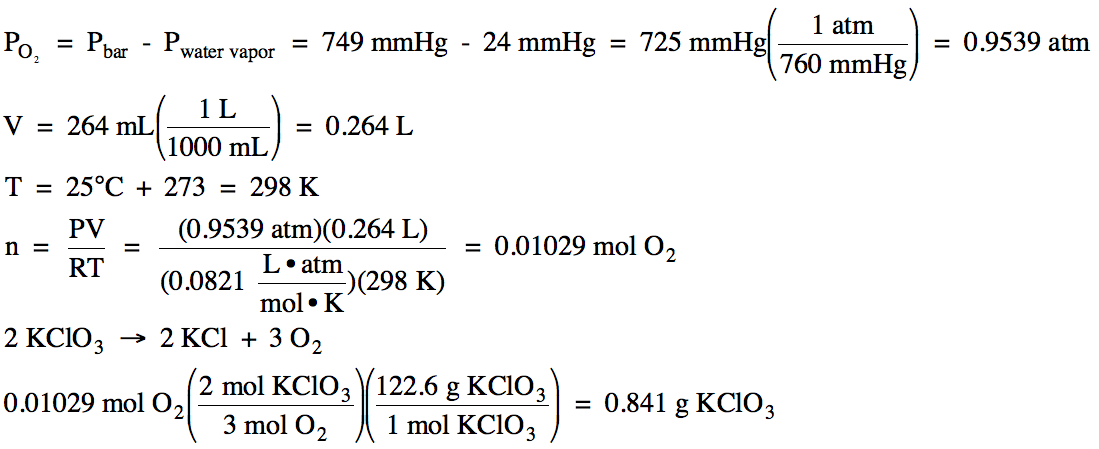
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chemistry and physics equations commonly include “R”, which is the symbol for the gas constant, molar gas constant, or universal gas constant.
The Gas Constant is the physical constant in the equation for the Ideal Gas Law:
- PV = nRT
P is pressure, V is volume, n is the number of moles, and T is temperature.
It’s also found in the Nernst equation relating the reduction potential of a half-cell to the standard electrode potential:
- E = E0 – lnQ
E is the cell potential, E0 is the standard cell potential, R is the gas constant, T is the temperature, n is the number of mole of electrons exchanged, F is Faraday’s constant, and Q is the reaction quotient.
The gas constant is equivalent to the Boltzmann constant, just expressed in units of energy per temperature per mole, while the Boltzmann constant is given in terms of energy per temperature per particle. From a physical standpoint, the gas constant is a proportionality constant that related the energy scale to the temperature scale for a mole of particles at a given temperature.
Units for the gas constant vary, depending on other units used in the equation.
One common value is 8.3145 J/mol·K.
You May Like: What Does Abiotic Mean In Biology
What Is R In Pv Nrt
4/5pVnRTRRRof the answer
PV = nRT. n = number of moles. R = gas constant = 0.08206 / T = temperature in Kelvins. P = absolute pressure in atm.
Furthermore, what is the value of R in the ideal gas law? 0.082057 L
Keeping this in consideration, what is r in PV nRT for mmHg?
The value of the gas constant ‘R‘ depends on the units used for pressure, volume and temperature. R = 0.0821 liter·atm/mol·K. R= 8.3145 J/mol·K. R = 8.2057 m3·atm/mol·K. R = 62.3637 L·Torr/mol·K or L·mmHg/mol·K.
What is the R constant?
The gas constant R is 8.314 J / mol·K. Convert the numerical value of R so that its units are cal / . A unit conversion table will tell you that 1 cal = 4.184 J.
The Individual Gas Constant
The Individual Gas Constant depends on the particular gas and is related to the molecular weight of the gas. The value is independent of temperature. The induvidual gas constant, R, for a gas can be calculated from the universal gas constant, Ru , and the gas molecular weight, Mgas:
R = Ru/Mgas
In the imperial system the most common units for the individual gas constant are ft lb/slug oR. In the SI system the most common units are J/kg K.
Unit conversion: 1 J/kg K = 5.97994 ft lb/slug °R, and 1 ft lb/slug °R = 0.167226 J/kg K.
The Individual Gas Constant for gases:
For full table – rotate the screen!
| Gas |
| 379 |
Recommended Reading: Does Elton John Have Biological Children
The Ideal Gas Equation
Before we look at the Ideal Gas Equation, let us state the four gas variables and one constant for a better understanding. The four gas variables are: pressure , volume , number of mole of gas , and temperature . Lastly, the constant in the equation shown below is R, known as the the gas constant, which will be discussed in depth further later:
Another way to describe an ideal gas is to describe it in mathematically. Consider the following equation:
The term \ is also called the compression factor and is a measure of the ideality of the gas. An ideal gas will always equal 1 when plugged into this equation. The greater it deviates from the number 1, the more it will behave like a real gas rather than an ideal. A few things should always be kept in mind when working with this equation, as you may find it extremely helpful when checking your answer after working out a gas problem.
- Pressure is directly proportional to number of molecule and temperature.
- Pressure, however, is indirectly proportional to volume.
Derivation Of The Ideal Gas Law
Gases are distinguished from other forms of matter, not only by their power of indefinite expansion so as to fill any vessel, however large, and by the great effect heat has in dilating them, but by the uniformity and simplicity of the laws which regulate these changes. James Clerk Maxwell
ADVERTISEMENT
The ideal gas law is one of the most fundamental equations in physical chemistry, and it has been independently derived through experimental analysis and theoretical extrapolation. Originally, the ideal gas law emerged as a combination of 4 other distinct mathematical expressions that relate various properties of a gas to one another. The four individual laws are: Charless law, Boyles law, Gay-Lussacs law, and Avagadros law.
Recommended Reading: Reversible Figure Ground Relationship
The Significance Of The R Constant
Poets say science takes away from the beauty of the stars mere globs of gas atoms. I, too, can see the stars on a desert night, and feel them. But do I see less or more? Richard P. Feynman
So what exactly is the universal gas constant? The other parameters in the ideal gas equation all seem to correspond to some physically significant variable pressure , volume , amount of a substance , and temperature . R however, does not seem to do this. As with many mathematical constants, the term R does not explicitly map onto some physical quantity, entity, or process. Instead, the parameter R represents a relationship that holds between some physical quantities, specifically the pressure and volume of a gas, and the temperature and amount of gas. Specifically, R is equal to the ratio PV/nT.
The exact numerical value of the gas constant actually varies with the chosen units. The numerical value of R as 8.3144598 is a result of the specific units we use. This value of R is a result of measuring the physical magnitudes of gases in the standard SI units. The standard SI units and their symbol for each parameter in the ideal gas equation are:
- Pressure Newtons
- Volume Meter
- Temperature Kelvin
- Amount of substance moles