Gas Constant In Chemistry
- In chemistry, the gas constant goes by many names, including the ideal gas constant and universal gas constant.
- It is the molar equivalent to the Boltzmann constant.
- The SI value of the gas constant is exactly 8.31446261815324 JK1mol1. Usually, the decimal is rounded to 8.314.
The Gas Constant is the physical constant in the equation for the Ideal Gas Law:
P is pressure, V is volume, n is the number of moles, and T is temperature. Rearranging the equation, you can solve for R:
R = PV/nT
The gas constant is also found in the Nernst equation relating the reduction potential of a half-cell to the standard electrode potential:
E is the cell potential, E0 is the standard cell potential, R is the gas constant, T is the temperature, n is the number of mole of electrons exchanged, F is Faraday’s constant, and Q is the reaction quotient.
The gas constant is equivalent to the Boltzmann constant, just expressed in units of energy per temperature per mole, while the Boltzmann constant is given in terms of energy per temperature per particle. From a physical standpoint, the gas constant is a proportionality constant that related the energy scale to the temperature scale for a mole of particles at a given temperature.
Units for the gas constant vary, depending on other units used in the equation.
Difference Between R And S Configuration
April 30, 2018 Posted by Madhu
The key difference between R and S configuration is that the R configuration is the spatial arrangement of R isomer, which has its relative direction of priority order in a clockwise direction whereas S configuration is the spatial arrangement of S isomer that has its relative direction of priority order in an anticlockwise direction. Here, the relative direction of the priority order is the descending order of priorities of substituents.
The R and S isomers are organic molecules having a chiral center, which is a carbon atom that has four different substituents attached to it. These substituents get listed according to their priority .
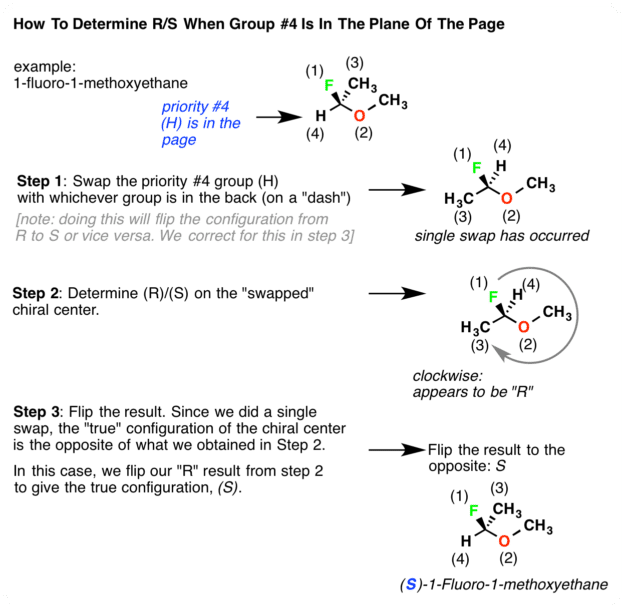
Determining R/s When The #4 Substituent Is In Front : A Short Cut
Lets first consider the molecule below. The name of this molecule is -1-fluoroethanol. It is listed below with priorities assigned based on atomic number. In this case F> O> C> H. So F is #1 and H is #4. The tricky part here is that the #4 priority is pointing out of the page .
How do we determine / in this case? There are two ways to do it.
Many instructors will tell you to simply rotate the molecule in your head so that the #4 priority is on a dash. Then you can assign R or S in the traditional way. This simple advice is not always an easy task for beginners.
Thankfully, it is technically unnecessary to perform such a mental rotation.
Heres a way around this. When the #4 priority is on a wedge you can just reverse the rules. So now we have two sets of rules:
If the #4 priority is on a dash:
If the #4 priority is on a wedge, reverse the typical rules:
- Counterclockwise = R
R and S can easily be assigned to either picture of the molecule. I still encourage you to use a model kit and learn how to do so, however. Organic chemistry is much easier to understand, and much more beautiful, if you can master how to visualize a tetrahedral carbon atom.
Read Also: Is Ap Physics 1 Hard
Orientation Of Given Molecule After Forth Group On Rear Side For Chiral Center:
Based on priority rules and molecular weight the priority of group is as follows:
- Hydrogen is forth priority group
- Methyl group is third priority group
- Hydroxyl group is first priority group
- Propyl group is second priority group
As here forth priority group is in the plane of paper so, double interchange will occur. H will change position with methyl and other two group interchange positions too.
Introduction To R/s Nomenclature
R and S nomenclature system is used to assign absolute configurations. This system replaced the old D and L system of nomenclature. R-S system is based upon certain rules devised by R. S. Cahn, Sir Christopher Ingold, and Vladimir Prelog in 1965. They firstly developed a ranking system to deal with the problem of absolute configurations at a chiral center. These rules are also known as Cahn-Ingold-Prelog rules or sequencing rules.
Recommended Reading: What Is Physics Good For
What Is Pro S And Pro R
Enantiotopic or diastereotopic pair of atoms or groups on a prochiral centre in a molecule is designated as pro-R and pro-S if replacement of one of them by an achiral ligand with higher priority than the other in the sense of CIP rule, without disturbing the priority of remaining ligands, convert the prochiral centre
How Do You Remember R And S Configuration
As opposed to this, if the arrow goes counterclockwise then the absolute configuration is S. As an example, in the following molecule, the priorities go Cl > N > C > H and the counterclockwise direction of the arrow indicates an S absolute configuration: So, remember: Clockwise R, Counterclockwise S.
You May Like: What Does The Word Sustainable Mean In Geography
Cahn Ingold And Prelog / Sequencing Rules
According to Cahn-Ingold-Prelog rules, the sequencing criterion should be based on priorities or rankings. A chiral atom always has four different substituents. All of those groups have been assigned priorities for knowing the exact direction of rotation of plane-polarized light, which will happen if the substance is made to go through with the experiment. These priorities are based on the shielding effects and electronic cloud densities, that atoms have, which become the cause for the rotation of planes.
Four substituents on chiral tetrahedral carbon atoms are ranked according to decreasing priority by the sequencing rules.
Value Of The Gas Constant
The value of the gas constant ‘R’ depends on the units used for pressure, volume and temperature. Prior to 2019, these were common values for the gas constant.
- R = 0.0821 liter·atm/mol·K
- R = 8.3145 J/mol·K
- R = 8.2057 m3·atm/mol·K
- R = 62.3637 L·Torr/mol·K or L·mmHg/mol·K
In 2019, the SI base units were redefined. Both Avogadro’s number and the Boltzmann constant were given exact numerical values. As a consequence, the gas constant also now has an exact value: 8.31446261815324 JK1mol1.
Because of the relatively recent definition change, use care when comparing calculations prior to 2019 because the values for R are slightly different before and after the redefinition.
Read Also: What Is The Value Of K In Physics
R And S When The Lowest Priority Is A Wedge
You have two options here:
Option one. Turn the molecule 180o such that the hydroxyl is now pointing towards you and the hydrogen is pointing away. This allows to have the molecule drawn as needed the lowest priority pointing backward as it is supposed to be for determining the R and S configuration:
Next, assign the priorities chlorine-number one, oxygen-two, carbon-three and the H as number four.
The arrow goes clockwise, therefore the absolute configuration is R.
The problem with this approach is that sometimes you will work with larger molecules and it is impractical to redraw the entire molecule and swap every single chirality center.
For example, look at biotin with all these hydrogens pointing forward. Not the best option to redraw this molecule changing all the hydrogens and keeping the rest of the molecule as it should be.
This is why we have the second approach which is what everyone normally follows.
Here, you leave the molecule as it is with the hydrogen pointing towards you. Continue as you would normally do by assigning the priorities and drawing the arrow.
The only thing you have to do at the end is change the result from R to S or from S to R.
In this case, the arrow goes counterclockwise but because the hydrogen is pointing towards us, we change the result from S to R.
Of course, either approach should give the same result as this is the same molecule drawn differently.
Assigning R And S Configuration: Steps And Rules
To assign the absolute configuration, we need to first locate the carbon with four different groups connected to it. These are called chirality centers .
In our molecule, we only have one carbon with four different groups and that is the one with the bromine and we are going to assign the absolute configuration of this chiral center.
For this, you need to follow the steps and rules of the Cahn-Ingold-Prelog system.
Step 1:
Give each atom connected to the chiral center a prioritybased on its atomic number. The higher the atomic number, the higher the priority.
So, based on this, bromine gets priority one, the oxygen gets priority two, the methyl carbon is the third and the hydrogen is the lowest priority-four:
Step 2:
Draw an arrow starting from priority one and going to priority two and then to priority 3:
If the arrow goes clockwise, like in this case, the absolute configuration is R.
As opposed to this, if the arrow goes counterclockwise then the absolute configuration is S.
As an example, in the following molecule, the priorities go Cl > N > C > H and the counterclockwise direction of the arrow indicates an S absolute configuration:
So, remember: Clockwise R, Counterclockwise S.
Now, lets see what would be the absolute configuration of the enantiomer:
And this is another important thing to remember:
All the chirality centers in enantiomers are inverted .
The lowest priority must point away from the viewer.
Read Also: What Is Concentration In Psychology
Absolute Configurations Of Perspective Formulas
Chemists need a convenient way to distinguish one stereoisomer from another. The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations ‘Râ or ‘ S â .
The rules for this system of stereochemical nomenclature are, on the surface, fairly simple.
Rules for assigning an R/S designation to a chiral center
1: Assign priorities to the four substituents, with #1 being the highest priority and #4 the lowest. Priorities are based on the atomic number.
2: Trace a circle from #1 to #2 to #3.
3: Determine the orientation of the #4 priority group. If it is oriented into the plane of the page , go to step 4a. If it is oriented out of the plane of the page go to step 4b.
4a: : a clockwise circle in part 2 corresponds to the R configuration, while a counterclockwise circle corresponds to the S configuration.
Summary R Vs S Configuration
The organic compounds having chiral centers have R and S configurations. The R and S isomers are the related molecules of these configurations, respectively. The basis of R and S configuration is the priority of the substituents attached to the chiral center. To sum up the comparison the difference between R and S configuration is that the R isomer has its relative direction of the priority order in a clockwise direction. And, in contrast, the S isomer has its relative direction of the priority order in an anticlockwise direction.
Reference:
1. Libretexts. Absolute Configuration: R-S Sequence Rules. Chemistry LibreTexts, Libretexts, 6 Nov. 2017. Available here 2. Hunt, Ian R. R/S Nomenclature. Available here 3. Absolute Configuration. Wikipedia, Wikimedia Foundation, 10 Apr. 2018. Available here
Image Courtesy:
1.CIP-RS configurationsBy Fabiuccio~enwikibooks at English Wikibooks via Commons Wikimedia
Recommended Reading: Geometry Distance And Midpoint Worksheet Answers
Y& r Spoilers Sally Spectra And Nick Newman Have Great Chemistry
Sally and Nick have had amazing chemistry from the start. Unfortunately, Adam believes that Nick only cares about Sally because of him. Nick seems to genuinely care about Sally and wants to have a relationship with her. However, between Adam and Victor Newman , Nick may never get to see where this relationship will go.
Victor could cause a rift between himself and Nick for trying to look up dirt on Sally. Nick has defended Sally against Victoria time and again. Does Victor think that Nick wont defend Sally against his attacks? Adam is very likely to defend Sally himself. Will Victor cause issues with both sons by going after a woman they both care about?
What About When #4 Is Not In The Back
That seems simple enough! Is that it?, you might ask.
Uh, no. As it happens, theres a few bumps in the road toward determining / once we get beyond the simple example above.
These trickier cases fall into three main categories.
Were not going to be able to fully address all of these issues in this post. But we can certainly deal with #1 and make some headway with #2. Well deal with #3 in a future post.
Don’t Miss: How Do You Find Displacement In Physics
Orientation Of Given Molecule After Forth Group On Rear Side For Chiral Center :
Based on priority rules and molecular weight the priority of group is as follows:
- Hydrogen is forth priority group
- Methyl group is third priority group
- Chlorine is first priority group
- Ethyl group is second priority group
As here forth priority group is pointing outside the plane of paper so, double interchange will occur. H will change position with methyl and other two group interchange positions too.
Determining Cip Priorities: Breaking Ties With The Dot Technique
The quick answer is to use the dot technique. Heres how it works. Lets do it for 4-ethyl-4-methyloctane, above.
2. If there is a tie, list the atoms attached to each of those dotted atoms in decreasing order of atomic number.
3. Compare each list, atom by atom. In our example, since C> H, takes priority over so the CH3 group is assigned priority #4.
4. If there is still a tie, move the dots to the highest ranking atom in the list . The dots are helpful because they help you to keep track of where you are, which can be important in complex examples.
5. In this case, we keep moving along the chain. By the way, if you ever reach the end of the chain without determining a difference, that means that the groups are identical and it isnt a chiral centre after all.
6. By this point we have enough information to assign /. Since priority #4 is in the front, we can also break out our opposite rule for good measure:
You May Like: Kendall Hunt Geometry Answer Key
Stereochemistry: R And S Notation
How do we number the carbon atoms in symmetrical molecules while giving R and S notation. For instance, in 2,3-Dibromobutane, there is an ambiguity over which is carbon C2 and carbon C3. Will we call it or ?
The current version of Nomenclature of Organic Chemistry IUPAC Recommendations and Preferred Names 2013 reads as follows:
P-14.4 NUMBERING
When several structural features appear in cyclic and acyclic compounds, low locants are assigned to them in the following decreasing order of seniority:
When there is a choice for lower locants related to the presence of stereogenic centers or stereoisomers, the lower locant is assigned to CIP stereodescriptors Z, R, M, and r that are preferred to E, S, P, and s, respectively, which are preferred to the non-CIP stereodescriptors cis, trans, or r , c, and t .
Therefore, the chirality center R receives the lowest locant and thus a systematic name is -2,3-dibromobutane and not 2,3dibromobutane.
However, your drawing shows -2,3-dibromobutane.
What Is S Configuration
S configuration is the spatial arrangement of S isomer. S isomer has a different arrangement unlike that of the R isomer of the same molecule. The letter S comes from the Latin word Sinister, and it means, Left-handed. Unlike R configuration, the S configuration has the anticlockwise direction of the substituents that is, from the highest priority to the lowest priority.
Read Also: What Is Emasculation In Biology
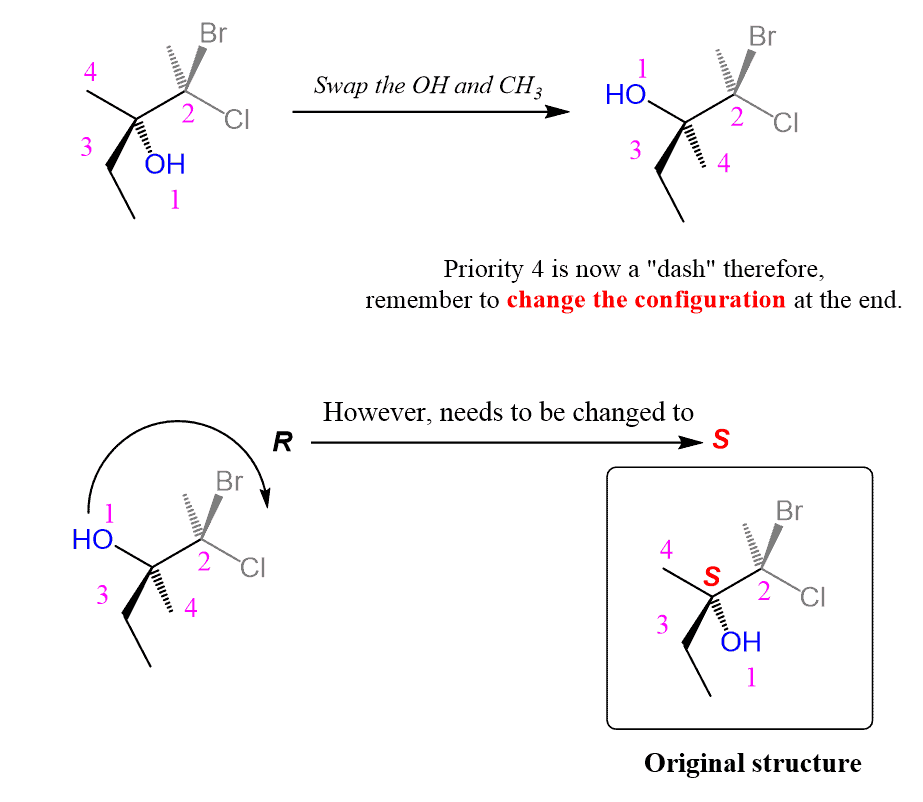
R And S When Group #4 Is Not A Wedge Or A Dash
There is a third possibility for the position of group 4 and that is when it is neither pointing away or towards you. This means we cannot determine the configuration as easily as if the lowest priority was pointing towards or away from us, and then switch it at the end as we did when group 4 was a wedge line.
As an example, what would be the configuration of this molecule?
For this, there is this simple yet such a useful trick making life a lot easier. Remember it:
Swapping any two groups on a chiral center inverts its absolute configuration :
Notice that these are different molecules. We are not talking about rotating about an axis or a single bond, in which case the absolute configuration must stay the same. We are actually converting to a different molecule by swapping the groups to make it easier determining the R and S configuration.
Lets do this on the molecule mentioned above:
The lowest priority group is in the drawing plane, so what we can do is swap it with the one that is pointing away from us . After determining the R and S we switch the result since swapping means changing the absolute configuration and we need to switch back again.
The arrow goes counterclockwise indicating S configuration and this means in the original molecule it is R.
Alternatively, which is more time-consuming, you can draw the Newman projection of the molecule looking from the angle that places group 4 in the back :