Historical Definition Of Oxidation Involving Oxygen
An older meaning of oxidation was when oxygen was added to a compound. This was because oxygen gas was the first known oxidizing agent. While the addition of oxygen to a compound typically meets the criteria of electron loss and an increase in the oxidation state, the definition of oxidation was expanded to include other types of chemical reactions.
A classic example of the old definition of oxidation is when iron combines with oxygen to form iron oxide or rust. The iron is said to have oxidized into rust. The chemical reaction is:
2 Fe + O2 Fe2O3
The iron metal is oxidized to form the iron oxide known as rust.
Electrochemical reactions are great examples of oxidation reactions. When a copper wire is placed into a solution that contains silver ions, electrons are transferred from the copper metal to the silver ions. The copper metal is oxidized. Silver metal whiskers grow onto the copper wire, while copper ions are released into the solution.
Cu + 2 Ag+ Cu2+ + 2 Ag
Another example of oxidation where an element combines with oxygen is the reaction between magnesium metal and oxygen to form magnesium oxide. Many metals oxidize, so it’s useful to recognize the form of the equation:
2 Mg + O2 2 MgO
Chem Oxidizing Reducing Agents
| Fe + Cd2+Fe2+ + Cd |
In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent.
Hey Natalie,
So oxidation involves a loss of electrons, while reduction involves a gain in electrons. This means that something undergoing oxidation would have a more positive charge, while something undergoing reduction has a more negative charge, relative to their original charges in the reaction.
Looking at the reaction, Fe is oxidized, as it goes from its original uncharged state to a Fe2+, indicating a loss of electrons. Contrastingly, Cd2+ becomes Cd, which means that it gained electrons, so Cd2+ is what is reduced.
An oxidizing agent is a species which causes something to become oxidized while being reduced itself. A reducing agent is a species that causes something to be reduced, while being oxidized itself. In this reaction, Cd2+ is the oxidizing agent, while Fe is the reducing agent.
Hope this helps!
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Also Check: New England Geography And Climate
Redox Reactions In Biology
Bottom: dehydroascorbic acid Enzymatic browning
Many important biological processes involve redox reactions. Before some of these processes can begin iron must be assimilated from the environment.
Cellular respiration, for instance, is the oxidation of glucose to CO2 and the reduction of oxygen to water. The summary equation for cell respiration is:
- C6H12O6 + 6 O2 6 CO2 + 6 H2O
The process of cell respiration also depends heavily on the reduction of NAD+ to NADH and the reverse reaction . and cellular respiration are complementary, but photosynthesis is not the reverse of the redox reaction in cell respiration:
- 6 CO2 + 6 H2O + light energy C6H12O6 + 6 O2
Biological energy is frequently stored and released by means of redox reactions. Photosynthesis involves the reduction of carbon dioxide into sugars and the oxidation of water into molecular oxygen. The reverse reaction, respiration, oxidizes sugars to produce carbon dioxide and water. As intermediate steps, the reduced carbon compounds are used to reduce nicotinamide adenine dinucleotide to NADH, which then contributes to the creation of a proton gradient, which drives the synthesis of adenosine triphosphate and is maintained by the reduction of oxygen.In animal cells, mitochondria perform similar functions. See the Membrane potential article.
What Is The Oxidizing Agent In The Following Reaction
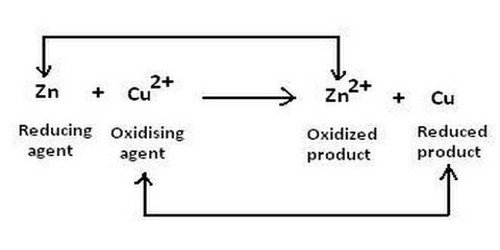
Which is the oxidizing agent in the following reaction?, Explanation: copper oxide is the oxidizing agent in this equation because it is responsible for hydrogen gas to form water.
Furthermore, How do you find the oxidizing agent?, So to identify an oxidizing agent, simply look at the oxidation number of an atom before and after the reaction. If the oxidation number is greater in the product, then it lost electrons and the substance was oxidized. If the oxidation number is less, then it gained electrons and was reduced.
Finally, What is an oxidising agent give example?, Oxidizing agents are those which oxidizes the Other compound and removes hydrogen from the compound. Oxidizing agents are substances that gain electrons. Examples of oxidizing agents include halogens, potassium nitrate, and nitric acid.
Read Also: What Does Consistent Mean In Algebra
Identify The Substance Oxidized Reduced Oxidizing Agent And A Reducing Agent For Each Of The Following Reactions: Pb + Pbo2 + 2h2so4 2pbso4 + 2h2o
Identify the substance oxidized reduced, oxidizing agent and a reducing agent for each of the following reactions:Pb + PbO2 + 2H2SO4 2PbSO4 + 2H2O
Oxidised substance:-PbReduced Substance:- PbO2Reducing agent:-PbOxidising agent:- PbO2Explanation:- Oxidising agent is a reagent which can increase the O.N. of an element in a given substance.Here, the Pb of PbO2 helps Pb to increase its oxidation number.The reduced substance is a reagent whose oxidation state has been decreased.Here, in this case, Pb in PbO2 has + 4 oxidation state and the O.N. is reduced to + 2 as Pb in PbSO4 in the product side is present in + 2 O.N.The oxidised substance is a reagent whose oxidation state has been decreased.Here, in this case, Pb has zero oxidation state and the O.N. is increased to + 2 as Pb in PbSO4 in the product side is present in + 2 O.N.Reducing Agent is a reagent which lowers the oxidation number of an element in a given substance.Here, Pb helps itself to decrease its oxidation number.
Miscellaneous Chemical Oxidizing Agents
Almost any oxidizing agent might be expected to oxidize an organoborane, but few have received systematic investigation. Moreover, aryldihydroxyboranes are relatively resistant to oxidation and nitration of the ring,94 and oxidation of a methyl group to a carboxy group with potassium permanganate95 has been achieved without breaking the ArB bond.
The reagent MoO5pyridineHMPA was introduced for the anhydrous, ambient temperature oxidation of intermediates in aldol reactions of alkenyloxyboranes.96 It also oxidizes simple organoboranes, the oxidation proceeding with retention of configuration of the alkyl groups.97
A mixture of ruthenium tetroxide, sodium periodate and sodium acetate was effective for the conversion shown in equation .98
The action of lead tetraacetate on trialkylboranes produces alkyl acetates 99
Although aryldihydroxyboranes appear to resist alkaline permanganate,94,95 butyldihydroxyborane is oxidized to butanol with this reagent.100
Dialkoxyalkylboranes are readily converted to monothioacetals by N-chlorosuccinimide in methanol .101
Sodium hypochlorite oxidizes aryldihydroxyboranes to phenols102 and trialkylboranes to the corresponding alcohols.103 Use of sodium hypobromite for the oxidation of phenyldihydroxyborane gave 2,4,6-tribromophenol, regardless of the ratio of the reactants.102
Alkyl- and phenyl-dihydroxyboranes, their anhydrides and dialkoxy derivatives are oxidized with alkyl hydroperoxides to the corresponding alcohols and phenols.105107
You May Like: 10 Branches Of Chemistry
List Of Oxidizing Agents
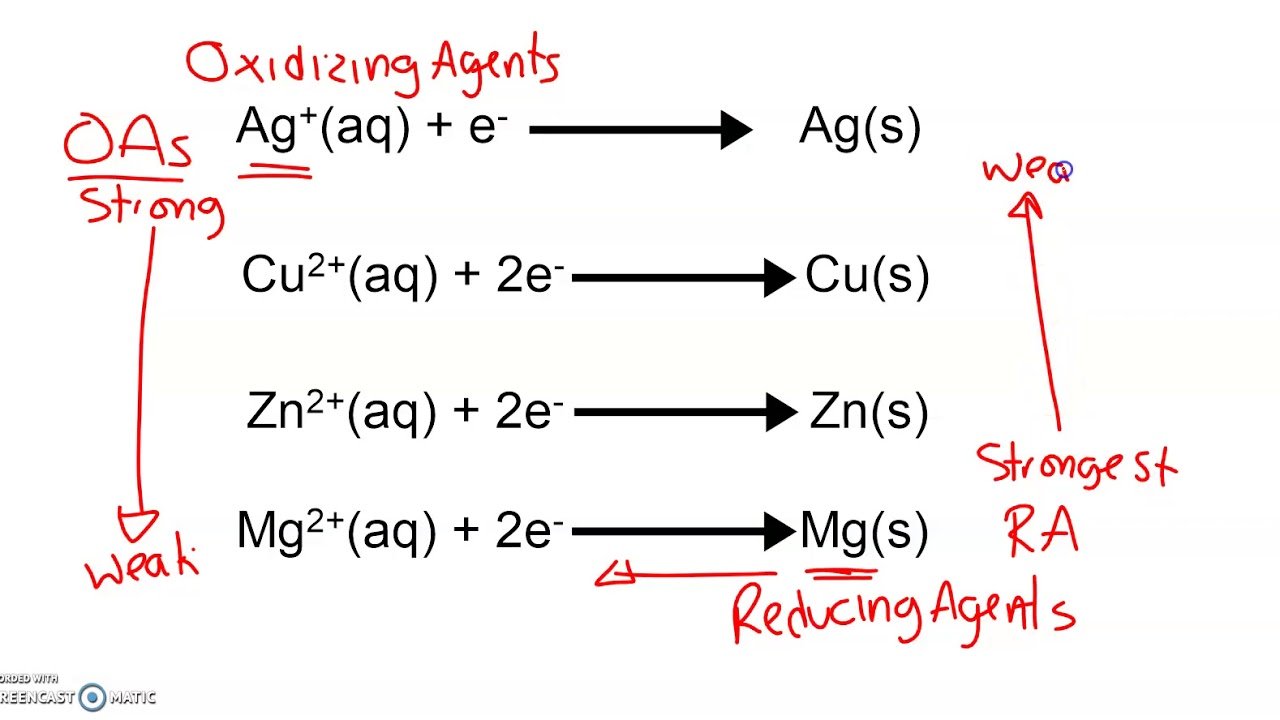
Strong Vs Weak Reducing Agent
The stronger the reducing agent, the weaker is the corresponding oxidizing agent. Fluorine gas is known to be a strong oxidizing agent and whereas F- is said to be a weak reducing agent. We also know that the weaker an acid then stronger is the conjugate base. Similarly, the weaker the oxidizing agent than the more strong is the corresponding reducing agent as shown in the figure below.
Also Check: Segment Addition Postulate Color By Number Worksheet Answer Key
The Dangerous Goods Definition Of An Oxidizing Agent:
An oxidizing agent, according to the dangerous goods definition, is a substance that can cause or contribute to the combustion of another substance. As a result, some materials classified as oxidizer by analytical chemists are not classified as oxidizing agents in the dangerous goods sense. One oxidizing agent example is potassium dichromate, which fails the oxidizin’s dangerous goods test.
Main Difference Reducing Agent Vs Oxidizing Agent
Reducing agents and oxidizing agents are chemical compounds involved in redox reactions. These compounds are the reactants of a redox reaction. The main difference between reducing agent and oxidizing agent is that reducing agent can lose electrons and be oxidized whereas oxidizing agent can gain electrons and be reduced.
Also Check: Beth Thomas Brother Now
What Are Oxidizing Liquids And Solids
Oxidizing materials are liquids or solids that readily give off oxygen or other oxidizing substances . They also include materials that react chemically to oxidize combustible materials this means that oxygen combines chemically with the other material in a way that increases the chance of a fire or explosion. This reaction may be spontaneous at either room temperature or may occur under slight heating. Oxidizing liquids and solids can be severe fire and explosion hazards.
Common oxidizing liquids and solids include:
- bromine
- peroxyacids
- persulphates
There are other chemicals that are oxidizing materials. For example, liquid air has been involved in many explosions because of its oxidizing properties. Liquid air itself has about 30% oxygen which makes it a powerful oxidant. However, when liquid air evaporates, it becomes richer in oxygen content when more volatile components evaporate slightly faster. Liquid nitrogen is safer and is preferred to liquid oxygen as a cryogenic liquid coolant.
It is wise to treat any unknown material, especially crystals in solvents known to form a peroxide , as very hazardous until it is positively identified.
What Are Reactive Groups
Reactive groups are categories of chemicals that typically react in similarways because they are similar in their chemical structure. Each substance witha chemical datasheet has been assigned to one or more reactive groups, andCAMEO Chemicals uses the reactive group assignments to make its reactivitypredictions. More info about reactivity predictions…
If you can’t find a chemical in the database–but you know what reactive groupit belongs in–you can add the reactive group to MyChemicals instead in orderto see the reactivity predictions.
There are 175 chemical datasheets assigned to this reactive group.
Read Also: How Old Is Elton Johns Kids
Definition Of Oxidizing Agent
For the reaction $\ce$, since oxidation state of $\ce$ increased from +4 in $\ce$ to +6 in $\ce$, can $\ce$ be referred to as the oxidizing agent?
- Ivan NeretinFeb 14 ’17 at 11:30
- $\begingroup$As for definitions, a reducing agent is an electron donor, an oxidising agent an electron acceptor.$\endgroup$
Oxidation means increase in Oxidation state or loss in electrons.
Example: $\ce$
Oxidizing agent is a substance that oxidizes other substances and reduces itself.
In your example, $\ce$ will act as oxidizing agent as in the reactant side its oxidation state is $0$ but in the product side its oxidation state is $-1$, which means that its oxidation state in decreased, i.e. $\ce$ is reduced.
Definition For Oxidizing Agent
Oxidizing agents can be defines in either of the 2 ways:
As an electron acceptor: they are chemical compounds whose atoms in a chemical reaction remove at least one electron from another atom. Oxidizing agents are reactants that undergo reduction in redox reactions, according to this definition.
As an atom-transfer substance: In a chemical process, an oxidizing agent is a substance that transfers at least one electronegative atom to a chemical species. Typically, the transferred atom is an oxygen atom. The transfer of an electronegative atom between two reactants is involved in a number of combustion processes and organic redox reactions.
The Fe2O3 molecule serves as an oxidant in the oxidising agent example above by transferring an electronegative oxygen atom to the carbon monoxide molecule.
Don’t Miss: Beththomas
Oxidation Reactions Of Reducing Agents
Followings are the types of reactions reducing agents undergo.
Oxidation of Zero Oxidation State into Positive Oxidation State
Lithium is a strong reducing agent because it readily loses an electron obtaining a +1 oxidation state. The half reaction would be,
Li Li+1 + e
Oxidation of a Positive Oxidation State into a Higher Positive Oxidation State
H2C2O4 is also a good reducing agent. The oxidation state of the C atom is +3. The highest oxidation state that the C atom can have is +4. Therefore, can oxidize into CO2. The half reaction is,
H2C2O4 2CO2 + 2H+ + 2e
Oxidation of a Negative Oxidation State into a Zero Oxidation State
O2 can be produced from O2- in oxides. For example, Ag2O can be oxidized into Ag and O2.
2Ag2O 4Ag + O2
Oxidation of a Negative Oxidation State into a Positive Oxidation State
Oxidation of H2S into H2SO4 cause the oxidation number of sulfur to change from -2 to +6.
S2- + 4H2O SO42- + 8H+ + 8e
What Colour Is Iodine In The Water
The complex formation changes the colour of the absorbed light. In water, an iodine solution is yellow-brown rather than violet.
Some other compounds of reducing agents include Carbon, Carbon monoxide, Ascorbic acid, Sulphur dioxide, Hydrogen, Oxalic acid, Phosphites, phosphorous acid, hypophosphites, etc.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Read Also: Which Is Harder Physics Or Chemistry
What Is A Reducing Agent
Reducing agent is an element that
-
Loses its electron/s to an electron recipient group and
-
Itself gets oxidized in a redox chemical reaction.
Thus, reducing agent reduces others while itself gets oxidized by losing electrons. As reducing agents lose electrons so generally, they possess low electronegativity and very small ionization energies. S-block metals generally work as good reducing agents. It is also called reductant or reducers.
Lets understand it by an example of redox reaction Image will be uploaded soon.
In the above reaction iron is losing 2 electrons thus, acting as a reducing agent. Oxidation state of iron as a reactant is 0 while +2 as a product in the reaction. Thus, the oxidation state of iron is increasing, so oxidation is taking place. While another reactant copper is gaining two electrons and working as an oxidizing agent. The oxidation state of copper is +2 as reactant in the reaction while 0 as product so reduction is taking place. Thus, iron is acting as a reducing agent but getting oxidized itself while copper is acting as an oxidizing agent but reduced.
|
Reducing Agent |
How To Identify The Oxidizing Agent
So to identify an oxidizing agent, simply look at the oxidation number of an atom before and after the reaction. If the oxidation number is greater in the product, then it lost electrons and the substance was oxidized. If the oxidation number is less, then it gained electrons and was reduced.So to identify an oxidizing agent, simply look at the oxidation number
that helps us keep track of electrons in an atom
Recommended Reading: Glencoe Geometry Concepts And Applications Practice Workbook Answer Key
Oxidation And Reduction Occur Together
Once the electron was discovered and chemical reactions could be explained, scientists realized oxidation and reduction occur together, with one species losing electrons and another gaining electrons . A type of chemical reaction in which oxidation and reduction occurs is called a redox reaction, which stands for reduction-oxidation.
The oxidation of a metal by oxygen gas could then be explained as the metal atom losing electrons to form the cation with the oxygen molecule gaining electrons to form oxygen anions. In the case of magnesium, for example, the reaction could be rewritten as:
2 Mg + O2 2
comprised of the following half-reactions:
Mg Mg2++ 2 e-
O2+ 4 e- 2 O2-
Solution For Problem 51p Chapter 4
Chemistry | 11th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
Chemistry | 11th Edition
Nitric acid is a strong oxidizing agent. State which of the following species is least likely to be produced when nitric acid reacts with a strong reducing agent such as zinc metal, and explain why: \.
Step 1 of 2
Here we have to explain which of the following species is least likely to be produced when nitric acid reacts with a strong reducing agent such as zinc metal.
The given species are,
It is known that nitric acid is a strong oxidizing agent and zinc is a strong reducing agent. When this two react with other than Zn metal will reduce nitric acid hence N will gain electron and the oxidation number of N must decrease. The oxidation number of N in nitric acid is +5 then the product which will form must have lower oxidation state than +5.
Hence in order to find out the species which is least likely to be produced when nitric acid reacts with zinc metal can be obtained by calculating the oxidation number of N in all the given species and the species which has higher oxidation number than +5 can not be the product of this reaction.
__________________
Don’t Miss: Unit 1 Test Study Guide Geometry Basics Gina Wilson