What Is The Chemical Formula Of Ozone
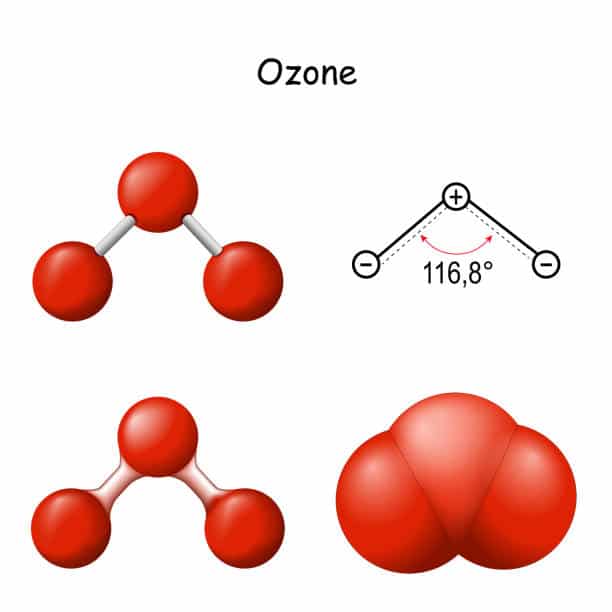
The formula of the chemical compound Ozone is O3
What is the formula for ozone?
Ozone, with the chemical formula O3, forms from ordinary oxygen in the upper stratosphere with the energy from the suns ultraviolet rays.
What are the properties of ozone?
Properties of ozone: Ozone in its pure state is blue in color which has a strong disturbing smell but in a limited proposition, it has a pleasant smell. It has the ability to absorb the UV rays which occupy the ultraviolet region which ranges between 220-290 nm of the atmospheric spectrum.
Just Remember That Ozone Is Good Up High Bad Nearby
Stratospheric ozone is good ozone . It forms about 10-30 miles above the Earth’s surface and forms a protective layer, called the ozone layer, that shields us from too much of the sun’s harmful ultraviolet radiation .
Ground-level ozone is bad ozone . Ozone harms human health and the environment when it forms close to the ground. The most significant things that cause ground-level ozone to form are:
- NOX and VOCs , and
We see higher ground-level ozone amounts most often in summer, due to increased amounts of UV radiation during the longer days, but ozone can still form in spring, fall, and even winter given the right conditions.
Even though the emission sources that contribute to ground-level ozone are typically found in urban areas, strong winds can also move it into rural areas, causing them to have high amounts of ground-level ozone.
People with respiratory conditions such as asthma, or those who are active outside on days when ozone amounts are high can feel shortness of breath, wheezing, and coughing. We can all take actions to help protect ourselves and reduce the damaging effects of this pollutant on our health and environment.
Distribution In The Stratosphere
| Learn how and when to remove this template message) |
The thickness of the ozone layer varies worldwide and is generally thinner near the equator and thicker near the poles. Thickness refers to how much ozone is in a column over a given area and varies from season to season. The reasons for these variations are due to atmospheric circulation patterns and solar intensity.
The majority of ozone is produced over the tropics and is transported towards the poles by stratospheric wind patterns. In the northern hemisphere these patterns, known as the Brewer-Dobson circulation, make the ozone layer thickest in the spring and thinnest in the fall. When ozone is produced by solar UV radiation in the tropics, it is done so by circulation lifting ozone-poor air out of the troposphere and into the stratosphere where the sun oxygen molecules and turns them into ozone. Then, the ozone-rich air is carried to higher latitudes and drops into lower layers of the atmosphere.
Read Also: What Does Eeg Measure In Psychology
What Is Good Ozone
Naturally occurring ozone in the stratosphere is sometimes referred to as good ozone. The stratospheric ozone layer absorbs ultraviolet radiation from the sun. This prevents most of the radiation from reaching the earths surface. It occurs through the interaction of solar ultraviolet radiation with molecular oxygen . This upper layer of ozone lives roughly 6 to 30 miles above the ground.
Its good ozone because it shields the planet from the suns harmful ultraviolet rays. Without this ozone in the stratosphere, the ultraviolet light would increase our chances of being sunburned and developing skin cancer.
Ozone Generators That Are Sold As Air Cleaners
There is a large body of written material on ozone and the use of ozone indoors. However, much of this material makes claims or draws conclusions without substantiation and sound science. In developing Ozone Generators that are Sold as Air Cleaners, the EPA reviewed a wide assortment of this literature, including information provided by a leading manufacturer of ozone generating devices. In keeping with EPA’s policy of insuring that the information it provides is based on sound science, only peer reviewed, scientifically supported findings and conclusions were relied upon in developing this document.
Several brands of ozone generators have EPA establishment number on their packaging. This number helps EPA identify the specific facility that produces the product. The display of this number does not imply EPA endorsement or suggest in any way that EPA has found the product to be either safe or effective.
Please Note: EPA does not certify air cleaning devices. The Agency does not recommend air cleaning devices or manufacturers. If you need information on specific devices or manufacturers, one resource you can consult is the Association of Home Appliance Manufacturers , 872-5955. AHAM conducts four certification programs for each category – room air cleaners, room air conditioners, dehumidifiers and refrigerator/freezers. The air cleaner certification program is known as AC-1.
Also Check: What Does Q Mean In Physics
Resonance Structures Of Benzene
Benzene is a very important aromatic hydrocarbon in organic chemistry. It has the chemical formula C6H6. The molecules of benzene have a cyclic structure consisting of alternating single and double bonds between adjacent carbon atoms. Each carbon atom is also bonded to one hydrogen atom. The two possible resonance structures of benzene are illustrated below.
The benzene molecule is stabilized by resonance, the pi electrons are delocalized around the ring structure. This delocalization causes each carbon-carbon bond to have a bond order of 1.5, implying that they are stronger than regular C-C sigma bonds. In the resonance hybrid of benzene, the delocalization of pi electrons is described with the help of a circle inside the hexagonal ring.
In benzene, Kekules first suggested two cyclohexatriene Kekule structures that have been taken together, they constitute the general structure as contributing structures. The hexagon replaces three double bonds in the hybrid structure on the right and represents six electrons in a collection of three molecular orbitals with a nodal plane in the molecule plane.
Thus, the resonance structures of some molecules and polyatomic ions are discussed briefly in this article. Learn more about this concept and other related concepts such as hyperconjugation, resonance effect, and electron dot formula.
Resonance Structures Of No3 Ion
Nitrogen is the central atom in a nitrate ion. It is singly bonded to two oxygen atoms and doubly bonded to one oxygen atom. The oxygen atoms that are singly bonded to the nitrogen hold a charge of -1 . The central nitrogen atom has a charge of +1 and the overall charge on the nitrate ion is -1. The three possible resonance structures of NO3 are illustrated below.
If a resonance hybrid of this polyatomic ion is drawn from the set of Lewis structures provided above, the partial charge on each oxygen atom will be equal to -. The net charge on the central atom remains +1. This resonance hybrid is illustrated below.
Read Also: Geometry Dash Full Apk Uptodown
Ozone Hole And Its Causes
The Antarctic ozone hole is an area of the Antarctic stratosphere in which the recent ozone levels have dropped to as low as 33 percent of their pre-1975 values. The ozone hole occurs during the Antarctic spring, from September to early December, as strong westerly winds start to circulate around the continent and create an atmospheric container. Within this polar vortex, over 50 percent of the lower stratospheric ozone is destroyed during the Antarctic spring.
As explained above, the primary cause of ozone depletion is the presence of chlorine-containing source gases . In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is dramatically enhanced in the presence of polar stratospheric clouds .
These polar stratospheric clouds form during winter, in the extreme cold. Polar winters are dark, consisting of three months without solar radiation . The lack of sunlight contributes to a decrease in temperature and the polar vortex traps and chills the air. Temperatures hover around or below â80 °C. These low temperatures form cloud particles. There are three types of PSC cloudsânitric acid trihydrate clouds, slowly cooling water-ice clouds, and rapid cooling water-ice cloudsâprovide surfaces for chemical reactions whose products will, in the spring lead to ozone destruction.
How Is Ozone Produced In The Atmosphere
When an oxygen molecule receive a photon , it dissociates into monoatomic atoms. These atoms attack an oxygen molecule to form ozone, O3.
The last reaction requires a third molecule to take away the energy associated with the free radical \ and \, and the reaction can be represented by
The over all reaction between oxygen and ozone formation is:
The absorption of UV B and C leads to the destruction of ozone
A dynamic equilibrium is established in these reactions. The ozone concentration varies due to the amount of radiation received from the sun.
The enthalpy of formation of ozone is 142.7 kJ / mol. The bond energy of O2 is 498 kJ / mol. What is the average O=O bond energy of the bent ozone molecule O=O=O?
Solution
The overall reaction is
Note that 3 O=O bonds of oxygen are broken, and 4 O-O bonds of ozone are formed. If the bond energy of ozone is E, then
\ & = 445 kJ / mol \end \]
DISCUSSIONThe ozone bonds are slightly weaker than the oxygen bonds. The average bond energy is not the bond energy for the removal of one oxygen from ozone.
Can the energy to remove one oxygen be estimated from the data given here?
The techniques used in this calculation is based on the principle of conservation of energy.
Example \
The bond energy of O2 is 498 kJ / mol. What is the maximum wavelength of the photon that has enough energy to break the O=O bond of oxygen?
Solution
The energy per O=O bond is:
The wavelength \ of the photons can be evaluated using
DISCUSSION
Recommended Reading: Can Mental Attitude Affect Biological Disease
Aerosols Sterilants And Carbon Tetrachloride
CFCs are used in aerosol products, as sterilants of medical equipment, and in a range of miscellaneous applications including food freezing, tobacco expansion, fumigation and cancer therapy. Carbon tetrachloride is used as a feedstock in the production of CFC-11 and CFC-12, in the production of key pharmaceuticals and agricultural chemicals, and as a catalyst promoter. CFCs and carbon tetrachloride are ozone depleting substances whose production and consumption is controlled under the Montreal Protocol. With support from the Protocol’s Multilateral Fund delivered by UNEP, UNDP, UNIDO, the World Bank and bilateral agencies, developing countries are phasing out these ozone depleting chemicals in this sector.
Solvents Coatings & Adhesives
In the past, CFC-113 use was essential in many industrial applications: in electronic assembly production processes, precision cleaning and general metal degreasing during manufacture, as well as in dry cleaning and other industrial applications. CFC-113 began to be used in the 1970s in metal degreasing and other areas owing to concern over the toxicity of the chlorinated solvents used previously. For many years 1,1,1-trichloroethane was the solvent of choice to replace other more toxic chlorinated solvents for general metal cleaning. Carbon tetrachloride is no longer used as a solvent in most countries because of its toxicity, but it is still used in some parts of the world.
CFC-113, 1,1,1-trichloroethane, CTC, and bromochloromethane are ozone depleting substances whose production and consumption is controlled under the Montreal Protocol. With support from the Protocol’s Multilateral Fund delivered by UNEP, UNDP, UNIDO, the World Bank and bilateral agencies, developing countries are phasing out these ozone depleting chemicals in this sector.
Also Check: What Is Statistical Significance In Psychology
How Is Ozone Measured In O3
Once inside an instrument, ozone can be measured by its absorption of ultraviolet light or by the electrical current produced in an ozone chemical reaction. The latter approach is used in the construction of ozonesondes, which are light- weight, ozone-measuring modules suitable for launching on small balloons.
How Does Atmospheric Ozone Affect Human Health
Ozone has two properties of interest to human health. First, it absorbs UV light, reducing human exposure to harmful UV radiation that causes skin cancer and cataracts. Second, when inhaled, it reacts chemically with many biological molecules in the respiratory tract, leading to a number of adverse health effects. This course addresses this second property.
Also Check: What Is Budding In Biology
Resonance Structures Of No2 Ion
In the nitrite ion, the bond lengths of both nitrogen-oxygen bonds are equal. The Lewis dot structures of NO2 highlight a difference in the bond order of the two N-O bonds. The resonance hybrid of this polyatomic ion, obtained from its different resonance structures, can be used to explain the equal bond lengths, as illustrated below.
The resonance hybrid of NO2 suggests that each oxygen atom holds a partial charge of magnitude -½. The bond length of the N-O bonds is found to be 125 pm.
How Is Ozone Harmful
The same chemical properties that allow high concentrations of ozone to react with organic material outside the body give it the ability to react with similar organic material that makes up the body, and potentially cause harmful health consequences. When inhaled, ozone can damage the lungs. Relatively low amounts can cause chest pain, coughing, shortness of breath and throat irritation. Ozone may also worsen chronic respiratory diseases such as asthma and compromise the ability of the body to fight respiratory infections. People vary widely in their susceptibility to ozone. Healthy people, as well as those with respiratory difficulty, can experience breathing problems when exposed to ozone. Exercise during exposure to ozone causes a greater amount of ozone to be inhaled, and increases the risk of harmful respiratory effects. Recovery from the harmful effects can occur following short-term exposure to low levels of ozone, but health effects may become more damaging and recovery less certain at higher levels or from longer exposures .
Table 1. Ozone Heath Effects and Standards
| Health Effects |
|---|
Read Also: What Are The Possible Benefits Of Studying Biology
What Is O3 Used For
Ozone is used in many industries. It is used for purifying air and drinking water, in industrial waste treatment, oils, bleaching and waxes, and to make other chemicals. Some examples of workers at risk of being exposed to ozone include the following: Outdoor workers in areas with high levels of ozone.
Ozone As A Greenhouse Gas
Although ozone was present at ground level before the Industrial Revolution, peak concentrations are now far higher than the pre-industrial levels, and even background concentrations well away from sources of pollution are substantially higher. Ozone acts as a greenhouse gas, absorbing some of the infrared energy emitted by the earth. Quantifying the greenhouse gas potency of ozone is difficult because it is not present in uniform concentrations across the globe. However, the most widely accepted scientific assessments relating to climate change suggest that the radiative forcing of tropospheric ozone is about 25% that of carbon dioxide.
The annual global warming potential of tropospheric ozone is between 918 and 1022 tons carbon dioxide equivalent/tons tropospheric ozone. This means on a per-molecule basis, ozone in the troposphere has a radiative forcing effect roughly 1,000 times as strong as carbon dioxide. However, tropospheric ozone is a short-lived greenhouse gas, which decays in the atmosphere much more quickly than carbon dioxide. This means that over a 20-year span, the global warming potential of tropospheric ozone is much less, roughly 62 to 69 tons carbon dioxide equivalent / ton tropospheric ozone.
Recommended Reading: First Day Of School Algebra 1 Activity
Ozone Depletion And Global Warming
Among others, Robert Watson had a role in the science assessment and in the regulation efforts of ozone depletion and global warming. Prior to the 1980s, the EU, NASA, NAS, UNEP, WMO and the British government had dissenting scientific reports and Watson played a role in the process of unified assessments. Based on the experience with the ozone case, the IPCC started to work on a unified reporting and science assessment to reach a consensus to provide the IPCC Summary for Policymakers.
There are various areas of linkage between ozone depletion and global warming science:
In 2019, NASA reported that there was no significant relation between size of the ozone hole and the climate change.
What Other Methods Can Be Used To Control Indoor Air Pollution
Of the three, the first approach is the most effective. This involves minimizing the use of products and materials that cause indoor pollution, employing good hygiene practices to minimize biological contaminants , and using good housekeeping practices to control particles.
The second approach outdoor air ventilation is also effective and commonly employed. Ventilation methods include installing an exhaust fan close to the source of contaminants, increasing outdoor air flows in mechanical ventilation systems, and opening windows, especially when pollutant sources are in use.
The third approach air cleaning is not generally regarded as sufficient in itself, but is sometimes used to supplement source control and ventilation. Air filters, electronic particle air cleaners and ionizers are often used to remove airborne particles, and gas adsorbing material is sometimes used to remove gaseous contaminants when source control and ventilation are inadequate.
See Additional Resources section below for more detailed information about these methods.
You May Like: What Do We Study In Psychology
Prospects Of Ozone Depletion
Since the adoption and strengthening of the Montreal Protocol has led to reductions in the emissions of CFCs, atmospheric concentrations of the most-significant compounds have been declining. These substances are being gradually removed from the atmosphere since peaking in 1994, the Effective Equivalent Chlorine level in the atmosphere had dropped about 10 percent by 2008. The decrease in ozone-depleting chemicals has also been significantly affected by a decrease in bromine-containing chemicals. The data suggest that substantial natural sources exist for atmospheric methyl bromide . The phase-out of CFCs means that nitrous oxide , which is not covered by the Montreal Protocol, has become the most highly emitted ozone-depleting substance and is expected to remain so throughout the 21st century.
According to the IPCC Sixth Assessment Report, global stratospheric ozone levels experienced rapid decline in the 1970s and 1980s and have since been increasing, but have not reached preindustrial levels. Although considerable variability is expected from year to year, including in polar regions where depletion is largest, the ozone layer is expected to continue recovering in coming decades due to declining ozone-depleting substance concentrations, assuming full compliance with the Montreal Protocol.