Interesting Facts About Water:
- World water day is celebrated on 22nd March every year
- Earth consists of 78% water, rest constitutes for land
- 60% of your body is made up of water on an average, Jellyfish has over 95%
- 97% of water on earth is salt water
- 0.01% of the water available is fresh water
- More than 69% of freshwater is trapped in glaciers
Water Helps Your Body Remove Waste
Adequate water intake enables your body to excrete waste through perspiration, urination, and defecation. Water helps your kidneys remove waste from your blood and keep the blood vessels that run to your kidneys open and filter them out, according to the National Kidney Foundation. Water is also important for helping prevent constipation, points out the University of Rochester Medical Center. However, as research notes, there is no evidence to prove that increasing your fluid intake will cure constipation.
RELATED: Are You Drinking Enough Water? These Are the Health Risks of Dehydration
Why Do Animals Need Water
Animals need water in order for their bodies and brains to function properly because without water they will become dehydrated, malnourished and will ultimately not survive. Most animals actually gain the majority of their water from the food they eat, rather than consuming water by itself.
Proper water consumption ensures that an animal will maintain its body weight, body temperature and overall health. Unlike humans, animals do not have large quantities of stored water in their bodies, which means that they have to regulate their water intake carefully. While the camel can store and lose up to 40 gallons of water, not every animal is so lucky.
Read Also: Paris Jackson Dad
Chemical Reactions Of Water
Water is directly involved in many chemical reactions to build and break down important components of the cell. Photosynthesis, the process in plants that creates sugars for all life forms, requires water. Water also participates in building larger molecules in cells. Molecules like DNA and proteins are made of repetitive units of smaller molecules. Putting these small molecules together occurs through a reaction that produces water. Conversely, water is required for the reverse reaction that breaks down these molecules, allowing cells to obtain nutrients or repurpose pieces of big molecules.
Figure 4: Water acts as a buffer by releasing or accepting hydrogen atoms.
In conclusion, water is vital for all life. Its versatility and adaptability help perform important chemical reactions. Its simple molecular structure helps maintain important shapes for cells inner components and outer membrane. No other molecule matches water when it comes to unique properties that support life. Excitingly, researchers continue to establish new properties of water such as additional effects of its asymmetrical structure. Scientists have yet to determine the physiological impacts of these properties. Its amazing how a simple molecule is universally important for organisms with diverse needs.
Molly Sargen is a first-year PhD Student in the Biological and Biomedical Sciences Program at Harvard Medical School.
Water Is The Universal Solvent

As a polar molecule, water interacts best with other polar molecules, such as itself. This is because of the phenomenon wherein opposite charges attract one another: because each individual water molecule has both a negative portion and a positive portion, each side is attracted to molecules of the opposite charge. This attraction allows water to form relatively strong connections, called bonds, with other polar molecules around it, including other water molecules. In this case, the positive hydrogen of one water molecule will bond with the negative oxygen of the adjacent molecule, whose own hydrogens are attracted to the next oxygen, and so on . Importantly, this bonding makes water molecules stick together in a property called cohesion. The cohesion of water molecules helps plants take up water at their roots. Cohesion also contributes to waters high boiling point, which helps animals regulate body temperature.
Read Also: Who Is Prince Jackson’s Biological Father
Water Is An Excellent Solvent
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solventa substance capable of dissolving another substance. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. This is referred to as a sphere of hydration and serves to keep the particles separated or dispersed in the water. In the case of table salt mixed in water, the sodium and chloride ions separate, or dissociate, in the water, and spheres of hydration are formed around the ions. A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded by the partially positive charges of hydrogen atoms in water molecules. These spheres of hydration are also referred to as hydration shells. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems.
Ph Buffers Acids And Bases
The pH of a solution indicates its acidity or alkalinity.
You may have used litmus or pH paper, filter paper treated with a natural water-soluble dye for use as a pH indicator, tests how much acid or base exists in a solution. You might have even used some to test whether the water in a swimming pool is properly treated. In both cases, the pH test measures hydrogen ions concentration in a given solution.
Hydrogen ions spontaneously generate in pure water by the dissociation of a small percentage of water molecules into equal numbers of hydrogen ions and hydroxide ions. While the hydroxide ions are kept in solution by their hydrogen bonding with other water molecules, the hydrogen ions, consisting of naked protons, immediately attract to un-ionized water molecules, forming hydronium ions . Still, by convention, scientists refer to hydrogen ions and their concentration as if they were free in this state in liquid water.
Read Also: Half Life Chemistry Equation
Water Helps Your Brain Function Optimally
Ever feel foggy headed? Take a sip of water. Research shows thatdehydration is a drag to memory, attention, and energy, per a small study on adult men from China published in June 2019 in the International Journal of Environmental Research and Public Health. Its no wonder, considering H2O makes up 75 percent of the brain, the authors point out. One reason for that foggy-headed feeling? Adequate electrolyte balance is vital to keeping your body functioning optimally. Low electrolytes can cause issues including muscle weakness, fatigue, and confusion, says Gabrielle Lyon, DO, a functional medicine physician in New York City.
Water As Living Environment
Water-based organisms such as fish require water to breathe, directly breathing the oxygen dissolved in water. Without a water supply, they could not access oxygen and would suffocate.
Water also helps insulate the living environment for these organisms. When the body of water is deep enough, the water keeps fish warm during the winter months, even when ice forms on the surface of the water.
Related Articles
You May Like: Core Connections Algebra Chapter 4
Water For Mental Performance
Got an exam or an interview coming up or even a hot date that you really want to impress? Make sure youre well hydrated, because water has a huge impact on your mood and cognitive performance. Maintaining a good level of hydration has been shown to improve memory, attention, problem-solving and coordination. Theres evidence that it helps us stay calmer under pressure and reduce anxiety. Its even been linked to happiness and a reduction in negative, depressive thinking. But perhaps the strangest fact, brought to you by researchers from the University of Twente in Holland, is that we do our best thinking when we desperately need to pee! Its a risky strategy, but maybe you should try downing five glasses of water before your next big life event as these participants did. Science says youll make better decisions!
A Few Fascinating Facts To Wet Your Appetite
- Water plays a massive part in our moods and in our bodys ability to release energy from food. Feeling tired, lethargic, a bit low? It could be something more serious, but theres a pretty good chance youre just dehydrated.
- Brain cells are 80% water. For an average person, a 2% reduction in body water levels is enough to cause a 20% decrease in mental and physical performance. Thats the same as drinking four shots of alcohol, or twice as much as suffering from a bad hangover !
- We say for an average person, because theres evidence that some athletes have managed to hack their metabolisms through intensive training. World-class sportsmen can often perform incredible physical feats while suffering from levels of dehydration that would debilitate lesser mortals. Legendary long-distance runner Haile Gebrselassie once dropped 9.8% of his body mass running the Dubai marathon a race that he won with a record-breaking time of 2:05:29. A similar study found that the top five finishers in South Africas gruelling Iron Man triathlon all shed around 8% of their body weights One even lost 11%!
- Thats even more incredible when you consider that the body is around 60 per cent water so losing 11% body mass means shedding almost twice that in terms of water content. Plus, if all this talk of athletic achievements is making you cast guilty looks at your running shoes, you can tell yourself youre not overweight just well hydrated.
Don’t Miss: How Many Biological Kids Does Steve Harvey Have
Why Is Water Important For Living Organisms
All living organisms require water for survival. For example, all oxygen-dependent organisms need water to aid in the respiration process. Water has many uses for organisms. The way that it is utilized can be categorized in four different ways: as a solvent, as a temperature buffer, as a metabolite and as a living environment.
TL DR
Living organisms need water to survive. Many scientists even believe that if any extra-terrestrial exists, water must be present in their environments. All oxygen-dependent organisms need water to aid in the respiration process. Some organisms, such as fish, can only breathe in water. Other organisms require water to break down food molecules or generate energy during the respiration process. Water also helps many organisms regulate metabolism and dissolves compounds going into or out of the body.
Why Do Plants Need Water
March 25, 2014 By Emma Vanstone
A few days ago we noticed that one of our little pumpkin plants on the window sill had wilted. The soil looked dry so we watered the plant and after a few hours it was standing lovely and straight again, which got us wondering why do plants need water.
Plants need water to germinate.
We saw this with our bean in a jar. Water is needed to activate the process of germination, it also softens the seed making it easier for the plant to break through.
Photosynthesis is the process by which plants make energy to grow. Photosynthesis requires sunlight, water and carbon dioxide. We demonstrated what happens when a plant cannot photosynthesise when we made our cress caterpillar.
Nutrient transfer
Plants need water to absorb nutrients from the soil.
Transpiration
Transpiration is the process by which water moves up the stem of a plant from root to leaf when water is lost from the plant due to evaporation occurring at the leaves. This continual flow of water and nutrients keeps the plants cells firm, if the cells become short of water they lose firmness and the plant starts to wilt.
We can demonstrate transpiration by placing white flowers in coloured water, the water travels up the stem to the petals which become coloured like the water.
Have you ever noticed your plants wilting? Did they revive after you watered them?
Suitable for Key Stage 1 Living things and their habitats
Recommended Reading: Edgenuity Unit 1 Test Answers
Structure And Properties Of Water
You are likely already aware of some of the properties of water. For example, you know that water is tasteless and odorless. You also probably know that water is transparent, which means that light can pass through it. This is important for organisms that live in the water, because some of them need sunlight to make food by photosynthesis.
Water Cushions & Softens
Just as water in a waterbed has a cushioning effect with any movement, so it also has when buried in the earth. This cushioning is good protection during an earthquake, proven in seismic studies, when the groundwater slows down seismic waves and dampens their effects.
Water also softens the soil, making it easier for rain to percolate through to refill the aquiferthe earth’s underground storage space. When stored groundwater is sucked up and not replaced, the soil gradually condenses and becomes hard. Then water slides off the top, instead of being absorbed, and the earth loses its storage place and its shock protector.
Where the earth is receptive, rainwater sinks down through it to be stored in the aquifer.
Susette Horspool, CC-BY-SA 3.0
The softening effect of water is also evident in the way it prepares seeds to grow. Many seeds have hard covers that keep them from growing until water is present. Water softens the seed cover enough for the little shoots to break out, then the soft soil, mixed with organic matter, provides a perfect medium for the shoots to grow into full-fledged plants.
Without water most seeds would be too hard to grow, and the ground would be too hard or sandy to absorb and hold rain. Without water storage, droughts would kill, and earthquakes would be severe.
Recommended Reading: Fsa Algebra 1 Eoc Practice Test Answers
It Regulates Your Body Temperature
Staying hydrated is crucial to maintaining your body temperature. Your body loses water through sweat during physical activity and in hot environments.
Your sweat keeps your body cool, but your body temperature will rise if you dont replenish the water you lose. Thats because your body loses electrolytes and plasma when its dehydrated.
If youre sweating more than usual, make sure you drink plenty of water to avoid dehydration.
of body weight during physical activity.
Hydration also affects your strength, power, and endurance.
You may be more susceptible to the effects of dehydration if youre participating in endurance training or high-intensity sports such as basketball.
Negative effects of exercise in the heat without enough water can include serious medical conditions, like decreased blood pressure and hyperthermia. Extreme dehydration can cause seizures and even death.
Biological Roles Of Water: Why Is Water Necessary For Life
figures by Daniel Utter
Water makes up 60-75% of human body weight. A loss of just 4% of total body water leads to dehydration, and a loss of 15% can be fatal. Likewise, a person could survive a month without food but wouldnt survive 3 days without water. This crucial dependence on water broadly governs all life forms. Clearly water is vital for survival, but what makes it so necessary?
You May Like: Mcgraw Hill Geometry Answer Key
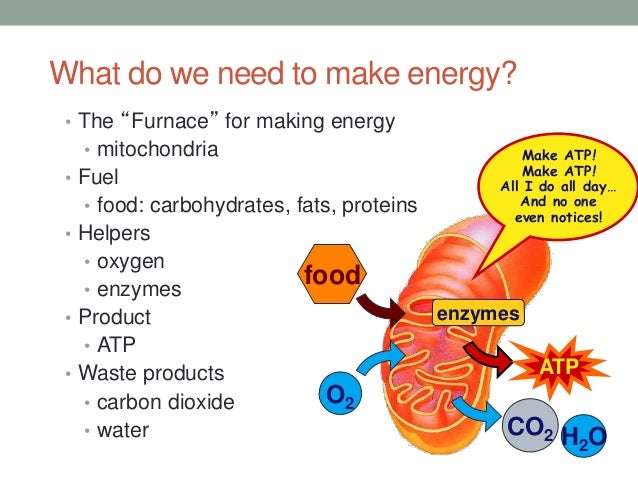
Water As A Metabolite
The sum total of chemical reactions within an organism is called metabolism. Water is a metabolite, or a chemical involved in reactions. In this way, it is is necessary for the continued survival of both plants and animals.
In plants, water aids in , the process by which plants convert sunlight into food. During the first stage of photosynthesis, water splits into hydrogen and oxygen atoms. Oxygen is released into the atmosphere, while hydrogen is used in the rest of the chemical reaction to produce glucose to feed the plant.
In animals, water aids in respiration. Water helps to split adenosine triphosphate into adenosine diphosphate and phosphoric acid. Cellular energy is released as a byproduct of this process. Water formation from oxygen and depleted hydrogen also moves waste products out of the body after the respiration cycle is complete.
Waters Heat Of Vaporization
Water also has a high heat of vaporization, the amount of energy required to change one gram of a liquid substance to a gas. A considerable amount of heat energy is required to accomplish this change in water. This process occurs on the waters surface. As liquid water heats up, hydrogen bonding makes it difficult to separate the liquid water molecules from each other, which is required for it to enter its gaseous phase . As a result, water acts as a heat sink or heat reservoir and requires much more heat to boil than does a liquid such as ethanol , whose hydrogen bonding with other ethanol molecules is weaker than waters hydrogen bonding. Eventually, as water reaches its boiling point of 100° Celsius , the heat is able to break the hydrogen bonds between the water molecules, and the kinetic energy between the water molecules allows them to escape from the liquid as a gas. Even when below its boiling point, waters individual molecules acquire enough energy from other water molecules such that some surface water molecules can escape and vaporize: we call this process evaporation.
Read Also: Cci4 Lewis Structure
When Is Water The Most Dense
Waters density is greatest at about 4 °C , in the liquid phase. Ice, waters solid phase, is more buoyant, so it forms at the surface of water bodies and freezes downward. Lakes and rivers rarely freeze completely, and the liquid water below can become a winter refuge for aquatic life. When ice melts in the spring, the slowly warming surface meltwater sinks, displacing the water below and mixing nutrients throughout the water column.
Earth & Air Without Water
Through precipitation , water in the air feeds the earth. Through evaporation, water in the earth feeds the air. What would happen if there were no water either place?
The sun, pouring down without moisture in the air, would beat on the earth and heat it up. The ground being rock, sand, or dry earth would have nothing in or growing on it to blunt the heat. Any carbon-based thing would burn up during the day. At night it would freeze.
There would also be nothing to soften the effect of volcanoes or to put out fires. There would be no cushioning effect against earthquakes. Any rubbing of tectonic plates against each other would be magnified far beyond what it is nowthe trembling would create massive rock slides and crumbling both at the site and in remote areas affected. The surface of the earth would burn and grind itself into dust.
Am I exaggerating? Most likely not. Read the link at the end of this article to learn about earthquakes and the softening effect of water underground.
Also Check: Did Michael Jackson Have Biological Children