Converting From Molarity To Normality
You can convert from molarity to normality using the following equation:
N = M*n
where n is the number of equivalents
To illustrate further, molarity and normality of some acids and bases are given below:
*Gram equivalent weight is determined by the amount of an ion that reacts, which could change depending on the reaction. Normality is not so straightforward as it will have different meanings depending on what you are dealing with:
- In acid-base chemistry, normality is used to express the concentration of protons or hydroxide ions in a solution.
- In redox reactions, the equivalence factor describes the number of electrons that an oxidizing/reducing agent can accept/donate.
- In precipitation reactions, the equivalence factor measures the number of ions which will precipitate in a given reaction.
What Is The Relation Between Normality And Molarity Of A Solution
Answer
Get Link in SMS to Download The Video
Aap ko kya acha nahi laga
Transcript
hello the question is what is the relation between normality and molarity of a solution this we have to calculate or find out ok we know the basic basic formulas of normality and molarity what is it the normality is an equivalent / volume in l ok is nearly as equation 1 ok and what is the molarity molarity is equal to moves / volume in l ok this is our equation to ok now we know we know that what is the relation between an equivalent and and and equivalent is equal to an end to end factor and into n factor ok this is the relation between an equivalent and and and boys this is our question 3 sunao
substitute substitute equation 3 into into question 1 equation 1 ok so what we will get what we will get you will get this and is equal to and equal and equivalent is equal to an end to end factor / volume ok we know the disturb this term is equal to molarity so from equation to from equation to what we can write from equation to the can write that can write that and is equal to into Inspector ok I am into n factor so this is our normality normality
this is our molarity molarity this is our n factor and factors ok this is the relation between normality and molarity I hope you understand the concept behind it thinking
Differences Between Normality And Molarity
Here are some key differences between normality and molarity.
| Normality | |
| It is defined as the number of gram equivalent per litre of solution. | It is defined as the number of moles per litre of solution. |
| It is used in measuring the gram equivalent in relation to the total volume of the solution. | It is used in measuring the ratio between the number of moles in the total volume of the solution. |
| The units of normality are N or eq L-1 | The unit of molarity is M or Moles L-1 |
You May Like: What Is Hplc Used For In Chemistry
Potential Issues Using N For Concentration
Although normality is a useful unit of concentration, it can’t be used for all situations because its value depends on an equivalence factor that can change based on the type of chemical reaction of interest. As an example, a solution of magnesium chloride might be 1 N for the Mg2+ ion, yet 2 N for the Cl- ion.
While N is a good unit to know, it’s not used as much as molality in actual lab work. It has value for acid-base titrations, precipitation reactions, and redox reactions. In acid-base reactions and precipitation reactions, 1/feq is an integer value. In redox reactions, 1/feq might be a fraction.
How Is Concentration Measured In Chemistry
First, it is necessary to understand how atoms and molecules combine in chemical reactions, which is not quite like the “ingredients” in, say a cooking recipe or a construction project. Ordinarily, you would keep track of different objects by measuring their masses or perhaps their volumes.
So, if you know that 1 kg of flour and 0.5 kg of sugar is enough to make 10 servings of a particular dessert product, you know that 100 kg of flour and 50 kg of sugar are enough for 1,000 servings. And in mixing drinks, if 50 mL of your own homemade fitness water needs 5 mL of vinegar, then 1,000 mL of the product needs 20 times this amount of of the vinegar reagent is 100 mL.
With reactions, it’s whole particles you need to count. These are measured in moles, and translating them into this “language” from mass only requires you to know the molar masses of the constituent atoms.
Read Also: What Is Location 5 Themes Of Geography
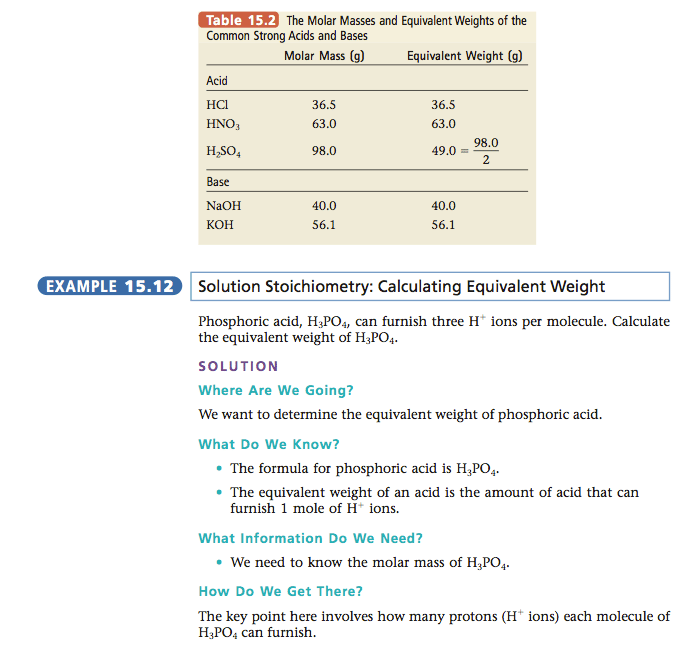
Normality Problems And Examples
Question 1. In the following reaction calculate and find the normality when it is 1.0 M H3PO4
H3AsO4 + 2NaOH Na2HAsO4 + 2H2O
Solution:
If we look at the given reaction we can identify that only two of the H+ ions of H3AsO4 react with NaOH to form the product. Therefore, the two ions are 2 equivalents. In order to find the normality, we will apply the given formula.
N = Molarity × number of equivalents
N = 1.0 × 2
Therefore, normality of the solution = 2.0.
Question 2. Calculate the normality of the solution obtained by dissolving 0.321 g of the salt sodium carbonate in 250 mL water.
Solution:
Question 3. What is the normality of the following?
a. N = 0.1381 mol/L × = 0.1381 eq/L = 0.1381 N
b. N = 0.0521 mol/L × = 0.156 eq/L = 0.156 N
Question 4. What will the concentration of citric acid be if 25.00 ml of the citric acid solution is titrated with 28.12 mL of 0.1718 N KOH?
Solution:
Na × =
Therefore, the concentration of citric acid = 0.1932 N.
Question 5. Find the normality of the base if 31.87 mL of the base is used in the standardization of 0.4258 g of KHP ?
Solution:
0.4258 g KHP × × :
= 2.085 × 10-3 eq base/0.03187 L = 0.6542 N
Normality of the base is = 0.6542 N.
Question 6. Calculate the normality of acid if 21.18 mL is used to titrate 0.1369 g Na2CO3?
Solution:
0.1369 g Na2CO3 × × × :
= 2.583 × 10-3 eq acid/0.02118 L = 0.1212 N
Normality of the acid = 0.1212 N.
Try this:
Question : What is the concentration of aluminium in a 3.0 M solution of aluminium sulfate?
Solved Question For You
Q: How can one differentiate Normality and Molarity?
Ans: Morality is the number of moles of solute per litre of solution, whereas Normality is the number of grams equivalent of solute per litre of solution.
Also, Molarity is a measurement of the moles in the total volume of the solution, whereas Normality is a measurement of the gram equivalent in relation to the total volume of the solution.
You May Like: Graphing Quadratic Functions Glencoe Algebra 1
Calculation Of Normality In Titration
Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of unknown concentration until the reaction approaches its neutralization. To find the normality of the acid and base titration:
N1 V1 = N2 V2
- N1 = Normality of the Acidic solution
- V1 = Volume of the Acidic solution
- N2 = Normality of the basic solution
- V3 = Volume of the basic solution
Why Is Molality Used Instead Of Molarity
Molality differs from molarity only in the denominator. While molarity is based on the liters of solution, molality is based on the kilograms of solvent. … Molality is used because its value does not change with changes in temperature. The volume of a solution, on the other hand, is slightly dependent upon temperature.
Also Check: Average Algebra 1 Regents Score
Give The Relation Between Normality And Molarity
In the kitchen, it may be okay to categorize solutions as weak or strong, but this is not enough in a laboratory. The concentration of a solution determines how the molecules in the solution will collide with each other and thus, it determines the conditions for equilibrium and the reaction rates. There are many ways to define the concentration of solutions. The most commonly used amongst them are normality and molarity.
How To Calculate Normality
The following are the steps to calculate the normality:
We may also express Normality as eq/L i.e. equivalent per litre.
Also Check: What Is Hydrostatic Pressure Biology
You Are Reading A Preview
Activate your 30 day free trial to continue reading.
Molarity is the amount of a substance in a certain volume of solution. Molarity is defined as the moles of a solute per liters of a solution. Molarity is also known as the molar concentration of a solution.Molality is a measure of the number of moles of solute in a solution corresponding to 1 kg or 1000 g of solvent. Normality is defined as the number of mole equivalents per liter of solution: normality = number of mole equivalents/1 L of solution.
Molarity is the amount of a substance in a certain volume of solution. Molarity is defined as the moles of a solute per liters of a solution. Molarity is also known as the molar concentration of a solution.Molality is a measure of the number of moles of solute in a solution corresponding to 1 kg or 1000 g of solvent. Normality is defined as the number of mole equivalents per liter of solution: normality = number of mole equivalents/1 L of solution.
What Is A Mole
A mole of anything, such as atoms, molecules, marbles or giraffes, is equal to 6.022 × 1023 individual instances of that thing. This happens to be the number of particles in exactly 12 grams of the most common form of the element carbon, number 6 on the periodic table of elements. The number of grams in 1 mole of a given element is under its symbol in its personalized “box” on the table.
To get the molar mass of a molecule, or the mass of 1 mol of those molecules, simply add the individual masses of the atoms, being sure to account for subscripts. So for a water molecule, H2O, you would add the mass of 1 mol O to the mass of 2 mol H to get about 18.015 g.
Recommended Reading: What Is Variability In Math
Relation Between Normality And Molarity
Molarity and Normality are related as follows:
For acids the normality can be calculated with the following formula:
Normality = Molarity x Basicity
To know the value for basicity, count the number of H+ ions an acid molecule can give.
For bases the normality can be calculated with the following formula:
Normality = Molarity x Acidity
To know the value for acidity, count the number of OH ions a base molecule can give.
Looking For A Way To Reinforce Your Students Understanding Of These Concepts Try This Quick Review
Molarity and Molality
Molarity is defined as the number of moles of solute per literofsolution.molarity = moles of solute/liters of solution
Molality is defined as the number of moles of solute per kilogramofsolvent.molality = moles of solute/kilograms of solvent
Although their spellings are similar, molarity and molality cannot be interchanged. Molarity is a measurement of the moles in the total volume of the solution, whereas molality is a measurement of the moles in relationship to the mass of the solvent.
When water is the solvent and the concentration of the solution is low, these differences can be negligible . However, when the density of the solvent is significantly different than 1 or the concentration of the solution is high, these changes become much more evident.
Example:Compare the molar and molal volumes of 1 mol of a solute dissolved in CCl4 .
For a 1 Molar solution, 1 mol of solute is dissolved in CCl4 until the final volume of solution is 1 L.
For a 1 molal solution, 1 mol of solute is dissolved in 1 kg of CCl4.1 kg of CCl4 Ã Ã = 629 mL CCl4
Normality
Normality is defined as the number of mole equivalents per literofsolution:normality = number of mole equivalents/1 L of solution
Like molarity, normality relates the amount of solute to the total volume of solution however, normality is specifically used for acids and bases.
How to calculate normality from molarity
Note:The normality of a solution is NEVER less than its molarity!
Also Check: Who Is The Father Of Chemistry Biology And Physics
What Is The Difference Between Molarity And Normality
0 Comments
Molarity and normality are two important and commonly used concentrations in chemistry that are measured using two different approaches. Both terms are used to indicate quantitative measurement of a substance. If you want to determine the amount of copper ions in a solution, it can be given as a concentration measurement. Molarity and normality are the types of concentration measurement.
What is Molarity? Molarity is the most commonly used method of concentration. It is expressed as the number of moles of solute dissolved per litre of solution. Therefore, the unit of the molarity is mol/L. Molarity is also known as molar concentration and is represented by M.
For example, a solution of 1M of sodium chloride dissolved in water has a molarity of 1M.
Number of moles of solute can be calculated by dividing mass by the molecular weight of the solute. For example, if you want to prepare a 1M of potassium sulphate solution, 174.26 g mol-1 of potassium sulphate should be dissolved in one litre of water.
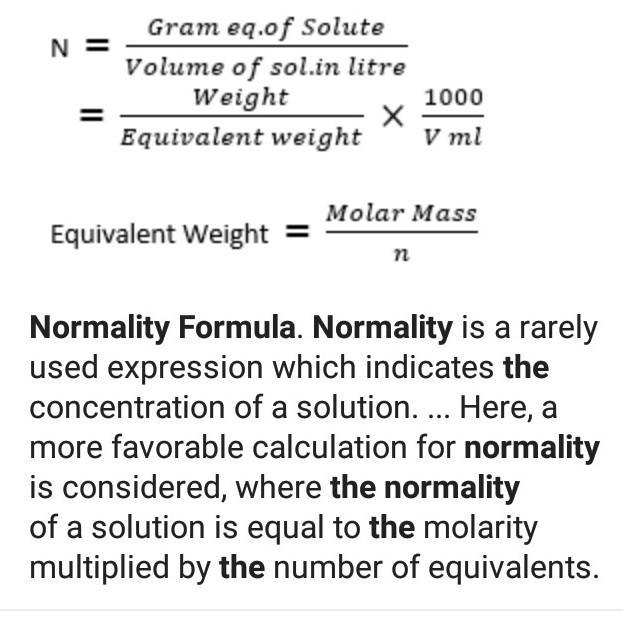
What is Normality? Normality is a measure of concentration that is equal to the gram equivalent weight of solute per litre of solution. Gram equivalent weight is a measure of the reactive capacity of a molecule*. Unit of normality is Eq/L. N is the symbol used to denote normality.
N = M*n
Formula Used To Find Out The Unknown Molarity Of The Solution Using Titration
M1V1= M2V2
- M1 is the molarity of titrant
- V1 is the volume of titrant used
- M2 is the molarity of the analyte
- V2 is the volume of the analyte solution
This formula is commonly used for titrimetric calculations and also for the standardization of solutions. Using this formula, we can find the unknown molarity of known solutions. During titration, we have two solutions. One of the solutions is with known volume but unknown molarity whereas, the second solution is of known molarity and unknown volume.
Read Also: Which Csu Has The Best Biology Program
Pls Briefly Explain The Concept Of Molarity Molality And Normality
Manisha answered this
MOLARITY
—Molarity is defined as the number of moles of solute per liter of solution.
molarity = moles of solute / liters of solution.
—Molality is defined as the number of moles of solute per kilogram of solvent.
molality = moles of solute / kilograms of solvent
Although their spellings are similar, molarity and molality cannot be interchanged. Compare the molar and molal volumes of 1 mol of solute dissolved in CCl4
—Normality is another ratio that relates the amount of solute to the total volume of solution.
It is defined as the number of equivalents per liter of solution:
normality = number of equivalents / 1 L of solution
Limitations In Using Normality
Many chemists use normality in acid-base chemistry to avoid the mole ratios in the calculations or simply to get more accurate results. While normality is used commonly in precipitation and redox reactions there are some limitations to it. These limitations are as follows:
- It is not a proper unit of concentration in situations apart from the ones that are mentioned above. It is an ambiguous measure and molarity or molality are better options for units.
- Normality requires a defined equivalence factor.
- It is not a specified value for a particular chemical solution. The value can significantly change depending on the chemical reaction. To elucidate further, one solution can actually contain different normalities for different reactions.
Read Also: What Are The Properties Of Colloids In Chemistry
Relationship Between Molarity And Normality
The molarity of a solution can be converted into normality using the number of equivalents of a solute present in a solution.
N = M x f
Where N is the normality,
M is the molarity,
f is the number of equivalents of solute.
The number of equivalents is the number of ions or groups of atoms released for a particular reaction.
How Normality Can Change
Because normality references concentration with respect to the reactive species, it’s an ambiguous unit of concentration . An example of how this can work may be seen with iron thiosulfate, Fe23. The normality depends on which part of the redox reaction you’re examining. If the reactive species is Fe, then a 1.0 M solution would be 2.0 N . However, if the reactive species is S2O3, then a 1.0 M solution would be 3.0 N .
Read Also: What Is N3 In Chemistry
How To Convert Molarity To Normality
The below equation will help you convert M to N.
N = M×n
n = the number of equivalents
For some chemicals, when n = 1, N and M are the same.
For the latest updates on Normality, Molarity, and Relation between normality and molarity register with BYJUS.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
How To Calculate Normality In Chemistry
When you hear the word “normality,” science may not be the first thing that comes to mind. “Normal,” to most people, means “ordinary” or “typical,” and doesn’t seem to have a whole lot of scientific weight. But in chemistry, the term normality is closely related to a few core chemistry concepts, and in particular, it is vital in the area of solutions chemistry.
If you see “normal” as “expected,” even in the chemical sense this is more or less on target: A normalized preparation is one that has been created in proportion or relation to an established standard. To discover how to calculate the normality of NaOH, or how to convert from normality to molarity , read on!
You May Like: What Is The Paradox Of Choice Psychology