Optical Properties Of Colloidal Solutions: Tyndall Effect
Colloids exhibit a phenomenon known as the Tyndall effect observed by Tyndall in 1869. When we pass an intense converging beam of light through a colloidal solution kept in dark, the path of the beam gets illuminated with a bluish light. This phenomenon of scattering of light by colloidal particles is called the Tyndall effect and the illuminated path is known as the Tyndall cone. The dispersed colloidal particles scatter the light falling on them resulting in emissions that are comparable to ultraviolet and visible radiations. These scattered radiations get illuminated.
The zone of scattered light is observed to be much larger than the particle itself. This makes the colloidal particles to appear as tiny bright spots when viewed under a microscope. This has to be done at right angles to the beam of light.
True solutions do not exhibit a Tyndall effect. This is because the size of particles present in a true solution are too small to scatter light. Thus, the Tyndall effect can be used to distinguish a colloidal solution from a true solution.
Learn different types of Emulsion and its properties here.
Kinetic Properties Of Colloids
During the observation of the colloidal dispersion under an ultra-microscope, it is clearly seen that the particles are in a continuous movement in the solution. This random zigzag movement of the particles in the colloidal solution is called the Brownian effect. This movement is mainly due to the unique bombardment of the molecules present in the dispersed medium on the colloidal particles.
To get more details on this topic you can download BYJUS The Learning App.
Tyndall Effect: The Optical Properties Of Colloidal Solutions
Colloids show a phenomenon known as the Tyndall effect, which John Tyndall identified in 1869. When we shine a bright converging beam of light through a dark colloidal solution, the path of the beam is lit with a blue glow. The scattering of light by colloidal particles is referred to as the Tyndall effect, and the lighted route is referred to as the Tyndall cone. The distributed colloidal particles scatter the light that falls on them, producing emissions similar to ultraviolet and visible radiations. These dispersed radiations are lit.
Read Also: What Is Genetics In Biology
Optical Properties Of Colloids
Tyndall Effect
-
When a beam of light is passed through a colloidal solution kept in dark, the path of the beam gets illuminated with blue colour.
-
This phenomenon is known as the Tyndall effect and the path is known as the Tyndall cone.
-
The Tyndall effect is due to the scattering of light by colloidal particles.
-
Tyndall effect is not exhibited by a true solution. This is due to the particles in the solution are too small to scatter light.
Brownian Movement: The Mechanical Properties Of Colloidal Particles
The Brownian movement is a highly significant characteristic of scattered particles in a colloidal solution. When a colloidal solution is examined via an ultramicroscope, the colloidal particles may be seen moving in a zigzag pattern. The colloidal particles are constantly bombarded from all directions by the moving molecules of the dispersion medium.
Thus, this gives momentum to the particles, causing them to travel ahead and collide with another particle. Collisions cause the colloidal particle to travel in a random zigzag pattern.
Read Also: What Is Big G In Physics
Electrical Properties Of Colloidal Solutions
The particles of the colloidal solution carry the same type of charge, while the dispersion medium carries an equal and opposite charge. The charge on the dispersion medium balances the charge on dispersed particles and the solution as a whole is electrically neutral.
The dispersed particles of a colloid repel each other since they carry similar charges and this prevents them from settling down thus maintaining the stability of the sol. Based on the nature of the charge, the colloidal sols may be classified as positively charged and negatively charged sols.
Classification Of Colloids On The Basis Of Physical States
- Solid Sols A colloidal system in which the particles of one solid substance is dispersed into the particles of another solid substance. e.g. Coloured Glass, Gemstones, Rock Salt, some alloys, etc.
- Sol When the dispersion medium is solid and the dispersion phase is liquid in a colloidal system, then it is termed as Sol. e.g. Paints, inks, gold sol, silver sol, muddy water, starch, etc.
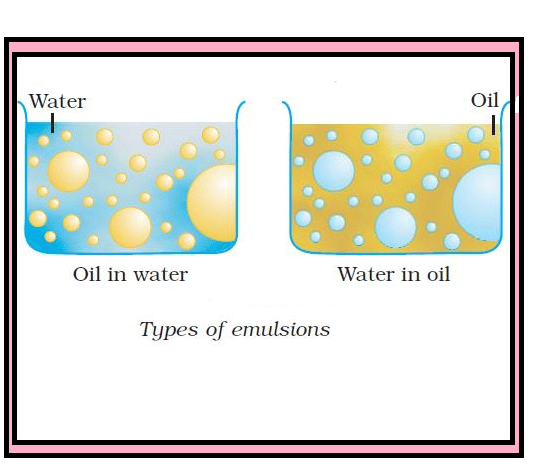
- Emulsion A fine dispersion of minute droplets of one liquid into another immiscible liquid is called Emulsion. The diameter of the droplets ranges from 10-4 to 10-6 cm. Milk, butter, cod liver oil, etc.
- Solid Aerosols A solution in which very minute fine particles of solid particles are dispersed into a gas is called solid aerosol. e.g. Smoke, dust storms, exhaust from industries and automobiles, etc.
- Gels A colloidal solution in which minute droplets of liquid are dispersed into a solid dispersion medium. e.g. jellies, Cheese, curd, shoe polish, etc.
- Solid Foam A colloidal solution in which minute particles of gas are dissolved into a solid dispersion medium. e.g. Pumice stone, rubber, cake, etc.
Also Check: What Is Aerobic Respiration In Biology
Properties Of Colloidal Solutions
A colloid is a mixture in which one substance of microscopically dispersed insoluble particles is suspended throughout another substance. Owing to this peculiar structure of colloid, it has varied physical and chemical properties. Let us explore more about the physical, chemical, optical as well as electrical properties of colloidal solutions.
Table of content
Arole Of Natural Chelating Ligands
The HS found in most natural waters absorb solar light in the range 300500 nm, and upon laser excitation three primary transient species were detected: triplet state , hydrated electron , and radical cation :
The results of steady-state irradiation suggested, however, that hydrated electrons are mostly trapped by molecular oxygen and their role in sunlight-irradiated natural surface waters is likely to be minor. Thus, the main species responsible for the photoinduced degradation of aquatic pollutants are triplet excited states of HS. The triplet state lifetime was estimated as longer than 2 s with an energy of 170 kJ mol 1. These parameters are adequate to generate the excited singlet O2 state , first of which is relatively low lying and thus can be easily populated by the energy transfer from the triplet states of most dyes the second O2 singlet state is more difficult to reach , but is much more reactive.
The 3HS may react with triplet O2 or with an oxidizable reactant by energy, hydrogen or electron transfer reactions . Direct reactions between 3HS and organic substrates seem less favored since the triplet state reaction with oxygen is very efficient . The quantum yield of singlet oxygen production determined at 365 nm as 13%, depends strongly on the irradiation wavelength and the HS nature .
Fig. 2. Reactions of humic substances in the excited triplet state with molecular oxygen and with organic reactants.
G. Sandri, … C. Caramella, in, 2016
Also Check: What Is No2 In Chemistry
Importance Of Tyndall Effect
The Tyndall effect has also been used to devise an instrument called utramicroscope .In this instrument, an intense beam of light is focussed on the colloidal solution contained in a glass vessel. The focus of light is then viewed with a microscope at right angles to the beam.
Individual colloidal particles appear as spots of bright light against a dark background.
Ultra microscope does not make the actual colloidal particles visible but only the light scattered by the colloidal particles can be seen through a microscope.
The colour of colloidal solutions depends on the wavelength of the light scattered by the dispersed particles. The wavelength further depends on the size and nature of the particles.
It has been observed that the colour of colloidal solutions also changes with the manner in which the observer receives the light.
For example: a mixture of milk and water appears blue when viewed by the reflected light but if transmitted light is viewed it is red. Similarly, gold sol is red in colour when the particles are fine but as the size of the particles increases, its colour changes to purple, then blue and finally golden.
Electrical Properties
The particles of the colloidal solutions possess electrical charge, positive or negative. The presence of charge is responsible for the stability of these solutions.
The sol particles carry some charge while the dispersion medium has no charge.
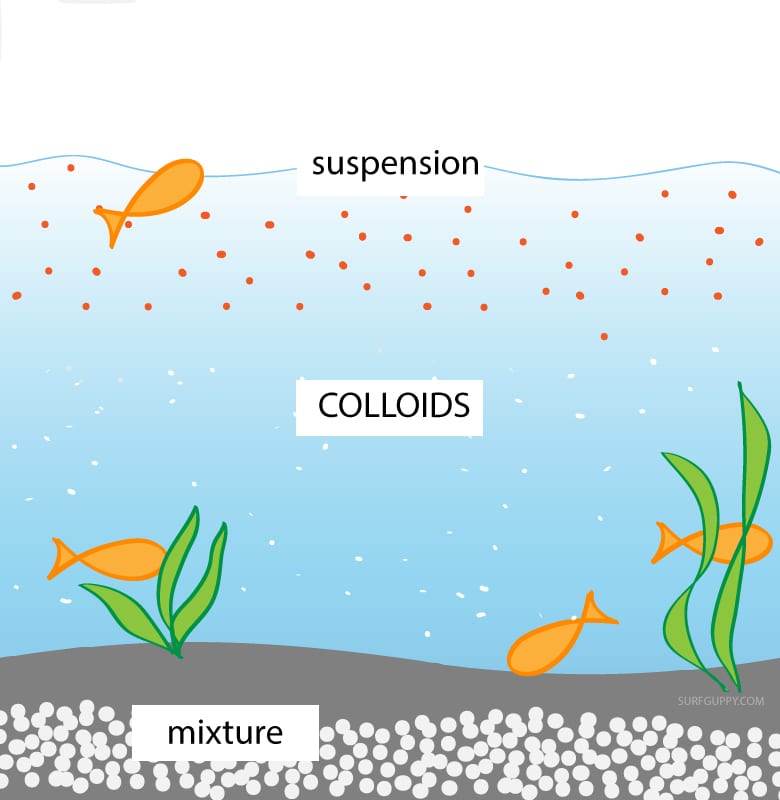
What Is The Difference Between Suspension And Colloid
Unlike colloid particles, those in a suspension can be separated by filtration. Colloids are capable of scattering light, but suspensions are not. Particles in a suspension can be seen with the naked eye, but those in a colloid require the use of a light microscope.
To follow more about the difference between suspension and colloid, download BYJUS The Learning App.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Recommended Reading: What Does M Stand For In Geometry
Colloids Meaning And Definition
In simple terms, we can define colloids as a mixture where one of the substances is split into very minute particles which are dispersed throughout a second substance. The minute particles are known as colloidal particles.
Alternatively, we can also say that colloids are basically solutions in which solute particle size ranges from 1nm 1000 nm. Colloids are heterogeneous in nature.
Important Considerations For The Design And Synthesis Of Spionsdrug Nanosystems
For drug delivery applications, it is important to ensure the SPIONsdrug nanosystem to have an adequate superparamagnetic property, a particular size suitable for its delivery, and a narrow size distribution for uniform biophysicochemical properties . A recent report showed that the particle size distribution could have a considerable effect on the hysteresis losses of the magnetic field amplitude . A wide particle size distribution would result in heterogeneous colloidal properties due to the wide range of blocking temperatures . In addition to particle size and its distribution, the magnetic properties of SPIONs are strongly related to impurity content of the particles, the polymer type, and the length of the polymeric shell. Furthermore, the concentrations of SPIONs in colloids are being recognized as having crucial importance. By increasing the concentration of magnetic NPs, a clustering of the particles may occur, leading to magnetic interactions and having a significant effect on the net magnetization .
Zofia Stasicka, in, 2011
Don’t Miss: Intermediate Algebra 5th Edition Ebook
What Is A Colloid Solution
A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. In a colloidal solution the particles are distributed evenly. During this process the particles do not settle down. This is one of the best known things about colloidal solutions.
Properties of colloids and their variation have been a well-known area ever since the primitive age. The best example to prove their familiarity with us is that we know from very early times that coagulation of milk results in the formation of curd.
What Is A Colloid
A Colloid is an intermediate between solution and suspension. It has particles with sizes between 2 and 1000 nanometers. A colloid is easily visible to the naked eye. Colloids can be distinguished from solutions using the Tyndall effect. Tyndall effect is defined as the scattering of light through a colloidal solution. The particles are termed as colloidal particles and the mixture formed is known as colloidal dispersion. Liquid, solid and gases all mix together to form a colloidal dispersion.
The different types of colloidal solution are:
- Aerosols: Solid or liquid mixed with gas Example: fog
- Sols: Solid mixed with liquid Example: Paint
- Emulsion: Liquid with liquid Example: oil and water
- Gel: liquid in solid Example: Fruit jelly
Read Also: What Is Implantation In Biology
Colloid Compared With Solution
A colloid has a dispersed phase and a continuous phase, whereas in a solution, the solute and solvent constitute only one phase. A solute in a solution are individual molecules or ions, whereas colloidal particles are bigger. For example, in a solution of salt in water, the sodium chloride crystal dissolves, and the Na+ and Cl ions are surrounded by water molecules. However, in a colloid such as milk, the colloidal particles are globules of fat, rather than individual fat molecules. Because colloid is multiple phases, it has very different properties compared to fully mixed, continuous solution.
High Concentration Biopharmaceuticals: Searching For High Colloidal And Conformational Stability
Although an appropriate formulation may be capable of overcoming poor colloidal properties by changing the conformational properties of a biopharmaceutical to alter its surfaces properties or by masking surface structural elements of the biopharmaceutical that are responsible for these poor properties, either directly or indirect, there are significant limitations for achieving such success. As a result, in developing a successful commercial biopharmaceutical, counting on formulation development alone to provide necessary colloidal and conformational stability is risky business.
A far safer and more rational approach is to invest some appropriate amount of time in trying to identify those biopharmaceutical candidates that intrinsically possess high colloidal and conformational properties from the start. Such work is often performed in late drug discovery or early in a molecule’s development and is typically referred to as a developability assessment . By embracing this activity one will more likely be in a position to avoid problems that may turn up in the development process that can place an enormously heavy burden on finding a formulation to correct what may not be achievable or may come at a great price, which could include drug failure from a prospective of the drug’s developability, manufacturability and stability.
Jeffrey Penfold, Ian M. Tucker, in, 2013
Also Check: What Is Temperature In Chemistry
What Are Colloidal Solutions And Their Properties
A colloidal solution is a type of mixture composed of particles varying in size from 1 to 1000 nanometres. The ions are uniformly dispersed in the colloidal solution. The particles do not calm down during this process.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Preparation Of Colloidal Systems
We can prepare a colloidal system by producing particles of colloidal dimensions and distributing these particles throughout a dispersion medium. Particles of colloidal size are formed by two methods:
A few solid substances, when brought into contact with water, disperse spontaneously and form colloidal systems. Gelatin, glue, starch, and dehydrated milk powder behave in this manner. The particles are already of colloidal size the water simply disperses them. Powdered milk particles of colloidal size are produced by dehydrating milk spray. Some atomizers produce colloidal dispersions of a liquid in air.
A colloidal gold sol results from the reduction of a very dilute solution of gold chloride by a reducing agent such as formaldehyde, tin chloride, or iron sulfate:
Some gold sols prepared in 1857 are still intact , illustrating the long-term stability of many colloids.
Read Also: Definicion De Literal En Algebra
Accelerating Methods For Shelf Life Prediction
The kinetic process of destabilisation can be rather long and it is often required for the formulator to use further accelerating methods in order to reach reasonable development time for new product design. Thermal methods are the most commonly used and consists in increasing temperature to accelerate destabilisation . Temperature affects not only the viscosity, but also interfacial tension in the case of non-ionic surfactants or more generally interactions forces inside the system. Storing a dispersion at high temperatures enables to simulate real life conditions for a product , but also to accelerate destabilisation processes up to 200 times.Mechanical acceleration including vibration, centrifugation and agitation are sometimes used. They subject the product to different forces that pushes the particles / droplets against one another, hence helping in the film drainage. However, some emulsions would never coalesce in normal gravity, while they do under artificial gravity. Moreover, segregation of different populations of particles have been highlighted when using centrifugation and vibration.
Classification Based On Properties Of Sol Particle
Classification of sols on the basis of properties are given as
Multi Molecular Colloids
When a dissolution occurs atoms or smaller molecules of substance aggregate together to form particles of colloidal dimensions. The particles thus formed are called multimolecular colloids.
In these sols, the dispersed phase consists of aggregates of atoms or molecules with a molecular size less than 1 atm. For example sols of gold atoms and sulphur molecules. In these colloids, the particles are held together by physical forces called Van der Waals forces. Metallic sols are usually multimolecular sols prepared by Bredigs are melted. These are usually lyophobic unstable, and separation is early possible.
Also Read:Van der Waals Equation
Macromolecular Colloids
These are substances having big size molecular called macromolecular which on demolition form size in the colloidal ran such substance are called macromolecular colloids. Thus macromolecule forming the dispersed phase are generally polymers having very high molecular masses.
Naturally occurring macromolecular are starch, cellulose proteins, enzyme gelatin etc. Artificial macromolecular and synthetic polymers such as nylon, polyester, plastics, polishers etc. They have usually lyophobic sols.
Recommended Reading: What Is Theorem In Math
Key Concepts And Summary
Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid, liquid, or gaseous medium. The particles of a colloid remain dispersed and do not settle due to gravity, and they are often electrically charged. Colloids are widespread in nature and are involved in many technological applications.
Classification Of Colloids On Properties Of Sol Particles
- Multimolecular Colloids: When smaller molecules of substance or many atoms get dissolution and combine to form a species whose size is in the range of colloidal size is known as multi-molecular colloids. e.g. sulfur solution with thousands of S8 particles.
- Macromolecular Colloids: The biomolecular such as enzymes or proteins which are bigger in size when immersed in a proper dispersion is known as macromolecular colloids. e.g. rubber, cellulose, starch, etc.
- Associated Colloids: There are certain substances whose molecules are diphilic in nature which means that the molecules of these particles contain a non-polar hydrophilic part and a polar hydrophobic part.
Also Check: What Does Pro Mean In Biology