Difference Between Molarity And Molality
Though the terms Molarity and Molality may seem somewhat similar as the two words sound almost the same, the sharp contrast between them and their usage makes it very important for the students to understand and to know, as it creates a huge difference while using it for various calculations. It may be difficult to notice and sometimes escape the eyes of the students, but there is one important distinction between the two terms Molarity and Molality.
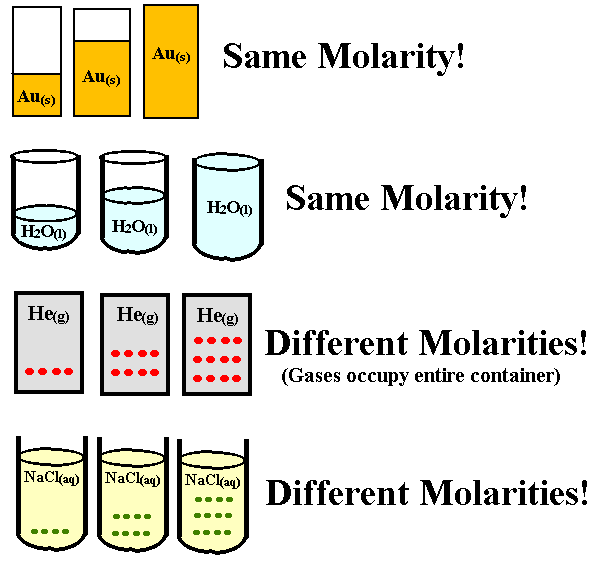
Molarity is the calculation of the number of solutes that are present in a liter of a solution. As we have mentioned earlier, a solution contains both the solute and the solvent. Whereas, Molality is the calculation of the number of solutes that are present in a kilogram of the solvent itself. As we already know, a solvent is something that dissolves the solute.
How To Calculate Dilutions Using Molarity
If you ever have to make a solution in the lab, or just want to pass your AP Chemistry exam, you are going to need to get used to molarities. One of the best uses of molarity is to calculate dilutions fast! In the lab, we usually only have a couple of solutions that are created at specific molarities. These solutions are called stock solutions.
A stock solution is a standardized solution of precisely known molar concentration that will be found in labs in large volumes
A stock solution of 2.0 M hydrochloric acid is easy to produce and can be stored for a long time. Usually, however, you would need lower concentrations of HCl, think like 0.1 M or so, to do your reaction. In order to create this lower concentration solution, you must dilute the stock solution by adding more solvent. In some experiments such as titrations, low concentration acids and bases are more effective as they are easier to control. Thankfully there is an easy way to calculate the needed dilutions, just use this equation:
M1& V1 refer to the volume and molarity of the stock solution, respectively. Usually, you will leave V1 as a variable as you are trying to find the volume of the solution you will need. V2& M2 refer to the molarity and volume of the solution you are trying to make. Let’s see an example to show how it would work in a lab:
First, create the 5M solution,
Find the amount of salt in grams needed
Moles of salt will be 5M*2L=10mols required
Use the equation M1V1=M2V2
5M=1M
So, to recap:
Why Is Molarity Important
The first records of the term molarity come from around 1930. It combines the word molar, an adjective that refers to moles, and the suffix ity, a suffix used to create abstract nouns that express a state.
Why do we care about molarity? Because it tells us how densely packed a substance is in a solution. Is there a lot of salt in the water or only a little bit? If you know the molarity of a solution, you can also determine the exact amount of substance that is dissolved in a solution.
You May Like: What Is The Definition Of Absolute Value In Math Terms
Concentration Of A Solution: Definition
Concentration of a solution is primarily reported in molarity or moles per liter. The abbreviation for molarity is M and the concentration units are mol/L.
The definition of molarity means that you can find the molarity of a solution if you know the total number of moles of the solute and the total volume of the solution. So, in order to calculate the concentration of a solution , you need to divide moles of solute by total volume.
Tips
-
Concentration formula: To find the molar concentration of a solution, simply divide the total moles of solute by the total volume of the solution in liters.
Relation To Apparent Molar Properties And Activity Coefficients

For concentrated ionic solutions the activity coefficient of the electrolyte is split into electric and statistical components.
The statistical part includes molality b, hydration index number h, the number of ions from the dissociation and the ratio ra between the apparent molar volume of the electrolyte and the molar volume of water.
Concentrated solution statistical part of the activity coefficient is:
- ln
Don’t Miss: Common Core Algebra 1 Unit 5 Review Answer Key
Example : Calculating Molar Concentrations
A 355-mL soft drink sample contains 0.133 mol of sucrose . What is the molar concentration of sucrose in the beverage?
Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. Per this definition, the solution volume must be converted from mL to L:
M=\frac}}=\frac}\times \frac}}}=0.375M
Check Your Learning
A teaspoon of table sugar contains about 0.01 mol sucrose. What is the molarity of sucrose if a teaspoon of sugar has been dissolved in a cup of tea with a volume of 200 mL?
Effect Of Temperature On Molarity
The volume of a substance will usually be affected by a change in temperature. Since it is based on the volume of solution, Molarity gets affected by temperature. According to this concept, when there is an increase in temperature, density will decrease. The volume increases when temperature increases and vice versa.
You May Like: Algebra With Pizzazz Did You Hear About Page 103
Key Concepts And Summary
Solutions are homogeneous mixtures. Many solutions contain one component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is one for which the solvent is water. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being molarity, defined as the number of moles of solute per liter of solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution.
How Do We Use Molarity In The Laboratory Or Classroom
Molarity is one of the most common units used to measure the concentration of a solution. Knowing the molarity of a solution is useful because by knowing it you not only know if it is diluted or concentrated but also the actual concentration. In a lab, a chemist must frequently prepare a given volume of solutions of a known molarity. The task is to calculate the mass of the solute that is necessary to reach the desired molarity. Chemists can use the molarity equation to solve for moles, and the result can then be converted to grams.
A variety of glassware is used to prepare solutions that can then be used to complete tests and experiments involving molarity. Among that glassware, graduated cylinders, volumetric flasks, volumetric pipets, and burets are often used to measure out specific volumes. Check out our recent Chemistry 101 article on how to measure liquids for science activities to learn more about the basics of effectively measuring liquids as you engage in scientific experimentation.
In the classroom setting, kits such as this molarity lab investigation kit offers students an introduction to the concept of molarity. Students can first try to make a solution with a specific molarity, demonstrating the importance of good laboratory technique. In the second set of experiments students can perform a titration on a known acid solution before using the concepts they have learned to identify the concentrations in three unknown solutions.
Don’t Miss: What Does It Mean To Be Biologically Human
How To Calculate Molality With This Molality Calculator
Let’s show on the example how to calculate molality:
Determining The Molar Concentration By Titration
Titration is a technique with which you can find the concentration of an unknown solution, based on its chemical reaction with a solution with a known concentration. This process is based on adding the titrant to a known quantity of the unknown solution till the reaction is complete. You can then determine the concentration of the analyte by measuring the volume of titrant used.
Follow these steps to find the molarity of an unknown solution with the titration method:
For ratios other than 1:1, you need to modify the formula.
Example: 35 ml of 1.25 M HCl acid is needed to titrate a 25 ml solution of NaOH. In that case, you can use the 1:1 formula because one mole of HCl reacts with one mole of NaOH. Then, multiply the molarity of the acid by the volume of the acid 1.25 * 35 = 43.75 and the result, by the volume of the base. The molarity of the base equals 43.75 / 25 = 1.75 M.
Read Also: What Are The 4 Major Goals Of Psychology
Converting From Molarity To Normality
You can convert from molarity to normality using the following equation:
N = M*n
where n is the number of equivalents
To illustrate further, molarity and normality of some acids and bases are given below:
*Gram equivalent weight is determined by the amount of an ion that reacts, which could change depending on the reaction. Normality is not so straightforward as it will have different meanings depending on what you are dealing with:
- In acid-base chemistry, normality is used to express the concentration of protons or hydroxide ions in a solution.
- In redox reactions, the equivalence factor describes the number of electrons that an oxidizing/reducing agent can accept/donate.
- In precipitation reactions, the equivalence factor measures the number of ions which will precipitate in a given reaction.
Solved Example Of Molarity

A solution prepared using 15 g of sodium sulphate. The volume of the solution is 125 ml. Find the molarity of the given solution of sodium sulphate.
Solution: The molecular formula for sodium sulphate is \.
The molecular formula for water is \.
The molecular mass of sodium sulphate is
The number of moles of sodium sulphate as per the given question is calculated by the formula,
Given that, the volume of the solution is 125 ml.
Expressing the above values in terms of litres,
Volume = \
Now, using the formula given below, calculate the molarity of the given solution.
Molarity = number of moles of solute/volume of solution in litres
Substituting the values, we get,
Molarity = \
The molarity of the given solution is 0.85M or 0.85 mol/L.
Recommended Reading: What Does S Stand For In Chemistry
Example : Determining The Volume Of Solution Containing A Given Mass Of Solute
In Example 3, we found the typical concentration of vinegar to be 0.839 M. What volume of vinegar contains 75.6 g of acetic acid?
First, use the molar mass to calculate moles of acetic acid from the given mass: \text\times \frac}}=\text
Then, use the molarity of the solution to calculate the volume of solution containing this molar amount of solute:
\text\times \frac}}=\text
Combining these two steps into one yields:
\text\times \frac}}\times \frac}}=\text
75.6\text}_}_\text\left\left=1.50\text
Check Your Learning
0.370 L
Faqs On Molality Formula
1. What is the advantage of using molality over molarity?
Molality is preferred and has an added advantage in certain cases when the solution is highly concentrated and the solvent used is not water. Molarity depends upon temperature, so in cases where the temperature of the solution changes, the density also changes, and in that case, the molality of a solution is better than molarity. Hence, in these cases, it is better to use molality to express the concentration of the solution, especially because the mass of solute and mass of solvent does not depend upon temperature, and hence, they do not change. To determine the boiling point, melting point, or boiling point elevation and freezing point depression, molality is always a better option to be used.
2. What are the differences between molality and molarity?
Molality and Molarity, for a dilute aqueous solution, do not vary much, but for concentrated solutions, they vary. The main points of differences between them are as follows:
-
Molarity depends upon the mass of solvent in the solution, while molarity depends upon the volume of the solution.
-
Molality is expressed as m, while molarity is expressed as M.
-
Unit of molality is moles/Kg, while that of molarity is moles/litre.
-
Molality does not depend on the change of temperature of the solution, but molarity is affected by the change of temperature of the solution.
3. Calculate the molarity of a solution prepared from 20.20 grams of NaOH in 5.00 kg of water.
Now,
You May Like: What Does Oh Mean In Chemistry
Another Example Of Calculating Molarity
Using a different compound, calcium chloride, we can calculate the molarity of a solution in the same way. Lets start with these values:
Following the same process outlined above, we can determine the molarity of this calcium chloride solution in a few simple steps. First, the volume must be converted from milliliters to liters.
Next, we convert grams of calcium chloride into moles.
Finally, we divide the number of moles by the volume of the solution.
Example : Determining The Mass Of Solute In A Given Volume Of Solution
How many grams of NaCl are contained in 0.250 L of a 5.30-M solution?
The volume and molarity of the solution are specified, so the amount of solute is easily computed as demonstrated in Example 2:
\begin\\ M& =& \frac}}\\ \text& =& M\times \text\\ \text& =& 5.30\frac}}\times 0.250\text=1.325\text\end
Finally, this molar amount is used to derive the mass of NaCl:
\text\times \frac}}=77.4\text
Check Your Learning
How many grams of CaCl2 are contained in 250.0 mL of a 0.200-M solution of calcium chloride?
5.55 g CaCl2
When performing calculations stepwise, as in Example 4, it is important to refrain from rounding any intermediate calculation results, which can lead to rounding errors in the final result. In Example 4, the molar amount of NaCl computed in the first step, 1.325 mol, would be properly rounded to 1.32 mol if it were to be reported however, although the last digit is not significant, it must be retained as a guard digit in the intermediate calculation. If we had not retained this guard digit, the final calculation for the mass of NaCl would have been 77.1 g, a difference of 0.3 g.
In addition to retaining a guard digit for intermediate calculations, we can also avoid rounding errors by performing computations in a single step . This eliminates intermediate steps so that only the final result is rounded.
You May Like: What Is Equivalence Point In Chemistry
Relation To Apparent Properties
Molality appears in the expression of the apparent volume of a solute as a function of the molality b of that solute :
- 1 ^}_=}}\left+}}}
For multicomponent systems the relation is slightly modified by the sum of molalities of solutes. Also a total molality and a mean apparent molar volume can be defined for the solutes together and also a mean molar mass of the solutes as if they were a single solute. In this case the first equality from above is modified with the mean molar mass M of the pseudosolute instead of the molar mass of the single solute:
- + ^}_=b_^}_+b_^}_+b_^}_+\ldots } ,
Advantages And Disadvantages Of Using Molarity
There are two big advantages of using molarity to express concentration. The first advantage is that it’s easy and convenient to use because the solute may be measured in grams, converted into moles, and mixed with a volume.
The second advantage is that the sum of the molar concentrations is the total molar concentration. This permits calculations of density and ionic strength.
The big disadvantage of molarity is that it changes according to temperature. This is because the volume of a liquid is affected by temperature. If measurements are all performed at a single temperature , this is not a problem. However, it’s good practice to report the temperature when citing a molarity value. When making a solution, keep in mind, molarity will change slightly if you use a hot or cold solvent, yet store the final solution at a different temperature.
You May Like: What Does Pt Stand For In Math
Effect Of Dilution On Molarity
To make a concentrated solution, it has to be diluted with the solvent its volume changes as a result. If a solution is diluted from V1 to V2, the molarity of the solution also changes. The changes are shown in the equation below
M1V1 = M2V2
Moles of solute in original Solution 1 = Moles of solute in diluted solution 2
In the above equation,
- M1= Initial Molarity of the solution
- V1 = The volume that is being transferred
- M2= Final Concentration of the solution
- V2 = Final total Volume of the solution
Relation To Other Compositional Quantities
In what follows, the solvent may be given the same treatment as the other constituents of the solution, such that the molality of the solvent of an n-solute solution, say b0, is found to be nothing more than the reciprocal of its molar mass, M0 :
- b
- i , }}}=}}}=}}}=M_}M_}}=M_}M_}},}
where i and j are subscripts representing all the constituents, the n solutes plus the solvent.
Read Also: What Year Do You Take Chemistry