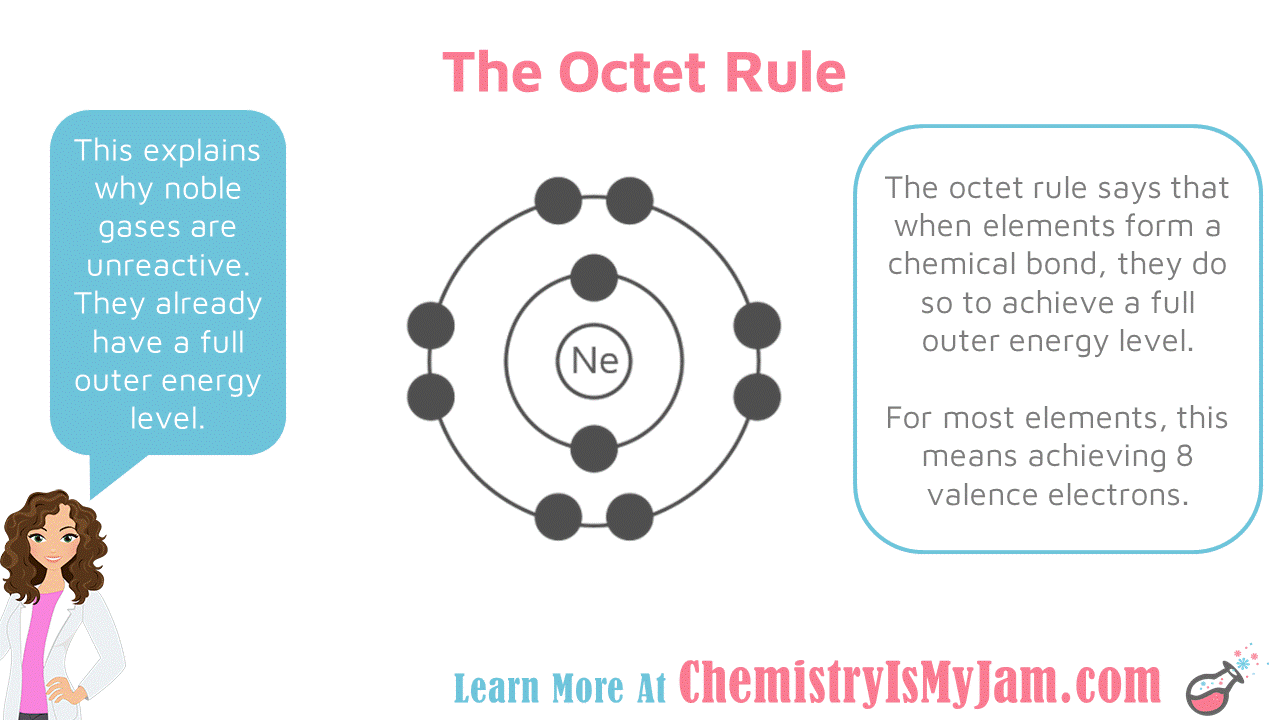
The Octet Rule Itself
Stability is considered to have been reached if the atom is enclosed by eight electrons, hence the name et. An octet can consist of both its own electrons and electrons that are shared. An atom will continue to form alliances until there are eight electrons, so until an octet is formed.
An is also known as a valence shell. An example of this would be CH4. The octet rule is considered unique enough to have its own theory because usually electrons will only form bonds in pairs, for example H2.
Once there are eight electrons within the valence shell and an , the atom then has the same electronic configuration as a noble gas.
Noble gasses – the elements found on the far right side of the periodic table – have no charge when filled with valence octets. These are configured as the most stable, full octet/no charge, and therefore have no reason to react and vary their configuration.
The rest of the elements do have a charge in the moment when they have eight electrons alone not shared. So they are always trying to gain, share or lose electrons, whatever they need to do, to become stable as a noble gas.
To sum up, atoms try to share electrons so that keeps charge to a minimum whilst creating an octet in a valence shell.
What Is Lewis Octet Rule
The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. Thus, an atom continues to form bonds until an octet of electrons is made. This is known as octet rule by Lewis.
What Is An Ionic Bond
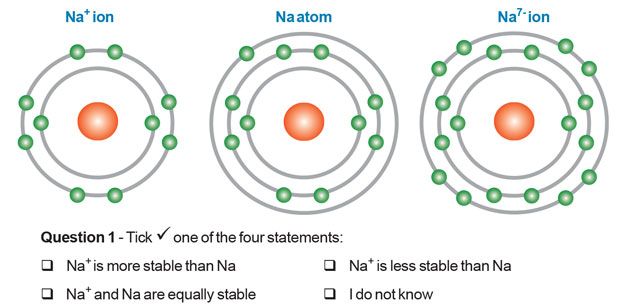
An ionic bond is a type of chemical bond that occurs between oppositely charged ions. An atom can either be positively or negatively charged, depending on the number of protons or electrons the atom has in excess. An ion is an atom with a net charge due to the gain or loss of electrons. Keeping in mind that electrons are negatively charged, if an atom gains an electron, it will have a negative charge, but if it loses an electron, it will have a positive charge. In short, an ion is an atom that contains a particular charge.
An ionic bond occurs between metals and non-metals, where one is electropositive and the other is electronegative. The electropositive metals lose an electron to become a positive ion, called a cation, whereas the electronegative nonmetals accept an electron and become a negatively charged ion, called an anion. The loss and acceptance of electrons is the same in order to form an ionic bond, and at the same time, each atom satisfies the octet rule!
Cation and anion.
So, when an atom has an unequal number of electrons and protons, it is called an ion. As a result, 2 atoms with unequal charges come together by either losing or gaining their electrons, thus attaining a neutral charge through an ionic bond. They remain bonded with the help of the attractive forces between them. The net result is that each atom is stable, as it has a completely filled valence shell with 8 electrons.
Ionic bonding in sodium chloride .
Also Check: What Is The Molecular Geometry Of Ccl4
Define Octet Rule And Write Its Significance And Limitations
The octet rule explains that atoms combine either by transfer of valence electrons from one atom to another or by sharing of valence electrons in order to have an octet i.e., eight electrons in their valence shells.Significance:It explains the reason for chemical combinations of atoms by ionic or covalent bonds.Limitations:1. Incomplete octet: When the central atom of certain molecules has less than 8 electrons in its valence shell and yet its stable.2. Expanded octet: When the central atom of certain molecules have more than 8 electrons and yet its stable.3. For Hydrogen to attain stability, it needs to share, gain or lose 1 electron. 4. Helium has only 2 electrons and is stable.
Was this answer helpful?
Deviations From The Octet Rule
A hypervalent molecule is a molecule that contains one or more main group elements that bear more than eight electrons in their valence levels as a result of bonding. Phosphorus pentachloride , sulfur hexafluoride , chlorine trifluoride , and the triiodide ion are examples of hypervalent molecules.
For the elements in the second period of the periodic table , the s2p6 electrons comprise the octet, and no d sublevel exists. As a result, the second period elements obey the octet rule without exceptions.
Phosphorus pentachloride
However, some of the third-period elements have been observed to bond to more than four other atoms, and thus need to involve more than the four pairs of electrons available in an s2p6 octet. This is possible because for n=3, the d sublevel exists, and it has five d orbitals. Although the energy of empty 3d-orbitals is ordinarily higher than that of the 4s orbital, that difference is small and the additional d orbitals can accommodate more electrons. Therefore, the d orbitals participate in bonding with other atoms and an expanded octet is produced. Examples of molecules in which a third period central atom contains an expanded octet are the phosphorus pentahalides and sulfur hexafluoride.
Sulfur hexafluoride
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
Boundless.
You May Like: Do You Capitalize Bachelor’s Degree In Psychology
General Chemistry/octet Rule And Exceptions
|
Appendices:Periodic Table ·Units ·Constants ·Equations ·Reduction Potentials ·Elements and their Properties |
The refers to the tendency of atoms to prefer to have eight electrons in the valence shell . When atoms have fewer than eight electrons, they tend to react and form more stable compounds. When discussing the octet rule, we do not consider d or f electrons. Only the s and p electrons are involved in the octet rule, making it useful for the representative elements . An octet corresponds to an electron configuration ending with s2p6.
What Is The Difference Between The Octet Rule And The Duet Rule
The rule of duet refers to the first five elements of the periodic table. They are most stable when the 1s orbital is filled with two electrons. Hydrogen looks to gain one electron, Lithium, Beryllium and Boron look to lose 1,2, or 3 electrons respectively in order to have a filled outer shell like Helium.
The rule of octet refers to the filling of the s and p orbital with eight electrons in order to become stable like a noble gas #s^2 p^6# .Elements look to lose or gain electrons in order to be like a noble gas. In doing so non-metals take on electrons and become negative anions while metals tend to lose electrons and become positive cations.
I hope this was helpful. SMARTERTEACHER
Read Also: How To Find Ksp From Solubility
What Does Octet Rule Mean
The octet rule states that atoms gain or lose electrons to attain an outer shell electron configuration nearest that of a noble gas. The attractive force between atoms is informally measured with this rule.
The octet rule dictates particular electron placement on the orbitals of the atom’s nucleus. It also determines whether electrons are added or lost through chemical reactions, and measures chemical reactivity of atoms based upon their specific electron configuration.
Example: Sodium And Chlorine
If a chlorine atom which has seven valence electrons, were to encounter a sodium atom which has just one valence electron, the chlorine atom would would remove the one valence electron from the sodium atom.
This in turn fills the valence shell of the chlorine atom, which would then take on the configuration of the nearest noble gas, which would be argon.
The sodium atom has now lost its one electron. It’s valence shell then becomes that of neon, also an element of noble gas.
This would be an Ionic example, the configuration of oppositely charged ions.
Don’t Miss: Algebra 1 Eoc Answer Key 2015
What Is The Lewis Electron Dot Structure
The Lewis structure is named after Gilbert N. Lewis, who first introduced the concept in his article, The Atom and the Molecule in 1916. Lewis structures only represent the valence electrons, which are identified as dots around the atom, each dot representing one valence electron. Most of the time, atoms with less than 8 electrons in their valence shell prefer to form compounds by either covalent bonding or ionic bonding.
The formation of such bonds can satisfy the octet rule. The elements in the second group of the periodic table fulfill the criteria for 8 electrons by losing, gaining or sharing electrons between atoms.
The type of bond that is formed depends on the number of electrons in the valence shell, as well as the total energy required to form the bond. The LEDS helps us identify how many free electrons are available for a chemical reaction.
Which Elements Do Not Meet The Octet Rule
There are some elements that do not adhere to the octet rule, among them we mention:
- Hydrogens that have a single orbital in the valence shell and for this reason can only accept two electrons.
- Boron that needs six electrons to be able to adhere to the rule, however it does not contain them.
- Aluminum that can achieve maximum stability with only six electrons.
- The beryllium that completes its last valence shell with only four electrons.
Recommended Reading: Kuta Angle Addition Postulate
Key Difference Octet Vs Duplet
There are chemically active and inactive atoms or compounds present in nature. This characteristic is mainly dependent on the number of electrons present in the outermost shells of the atoms. Atoms having incomplete shells may become active in order to complete their electron configuration to become stable. Atoms that are inactive have a complete electron configuration thus, these atoms do not react with any other atom unless in special conditions. Noble gases are always inactive in nature. Hence, they are known as inert gases. Inert gases are in the eighth column in the periodic table. Other elements in the same period tend to obtain the electron configuration of the inert gas in the end of that period, which is the most stable form in nature. Active atoms tend to complete the number of electrons according to . The key difference between octet and duplet is that while duplet is an atom having the maximum of two electrons in the outermost shell.
CONTENTS5. Summary
Solved Question For You
Q1. Which of the following is not true with regards to the octet rule?
A. It refers to an important chemical rule of thumbB. It refers to an important principle that the atoms which have bonded share eight outer electronsC. It states that the atoms like to have six electrons only in their full outer shellsD. Two notable exceptions to the octet rule are helium and hydrogen
A1. The correct option is option C., which is it states that the atoms like to have six electrons only in their full outer shells.
Download Toppr app for Android and iOS or signup for free.
Recommended Reading: Algebra 1 Eoc Test Answers
Why Is Multi Bonding Not Present In Phosphorus
An atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. This can only occur when the valence shell has enough orbitals to accommodate the extra electrons. For example, in the case of phosphorus, the valence shell has a principal quantum number n = 3.
Exception : Species With Odd Numbers Of Electrons
The first exception to the Octet Rule is when there are an odd number of valence electrons. An example of this would be Nitrogen Oxide also called nitric oxide . Nitrogen has 5 valence electrons while Oxygen has 6. The total would be 11 valence electrons to be used. The Octet Rule for this molecule is fulfilled in the above example, however that is with 10 valence electrons. The last one does not know where to go. The lone electron is called an unpaired electron. But where should the unpaired electron go? The unpaired electron is usually placed in the Lewis Dot Structure so that each element in the structure will have the lowestformal charge possible. The formal charge is the perceived charge on an individual atom in a molecule when atoms do not contribute equal numbers of electrons to the bonds they participate in.
No formal charge at all is the most ideal situation. An example of a stable molecule with an odd number of valence electrons would be nitric oxide. nitric oxide has 11 valence electrons. If you need more information about formal charges, see Lewis Structures. If we were to imagine nitric oxide had ten valence electrons we would come up with the Lewis Structure ):
Free Radicals
Read Also: Segment Addition Postulate Practice Answer Key
Explanation Of The Octet Rule
The octet rule refers to an important principle that the atoms which have bonded share eight outer electrons. This certainly means that the atoms valence shell has a resemblance with a noble gas.
The octet rule states that the atoms like to have eight electrons only in their full outer shells. For achieving eight electrons in their outer shells, atoms would gain or lose the valence electrons.
Furthermore, the atom does this by bonding with each other. Moreover, these atoms can be the same element or with different elements.
Two notable exceptions to the octet rule are helium and hydrogen. This is because both are happy with two electrons belonging in the outer shells.
Basic Concepts Of Chemical Bonding
- Explain why some elements can form an expanded octet
Key Points
- Main group elements that form more bonds than would be predicted by the octet rule are called hypervalent compounds, and have what is known as an expanded octet, meaning that there are more than eight electrons around one atom.
- The octet rule can be expanded by some elements by utilizing the d-orbitals found in the third principal energy level and beyond. Sulfur, phosphorus, silicon, and chlorine are common examples of elements that form an expanded octet.
- Phosphorus pentachloride and sulfur hexafluoride are examples of molecules that deviate from the octet rule by having more than 8 electrons around the central atom.
Terms
- hypervalent moleculeA molecule that contains an atom from a main group element which deviates from the octet rule by sharing more than eight electrons.
- expanded octetA case where an atom shares more than eight electrons with its bonding partners.
- main group elementElements that are not part of the transition metal block in the periodic table.
Read Also: Who Are Paris Jackson’s Biological Parents
What Is The Octet Rule
The octet rule dictates that atoms are most stable when their valence shells are filled with eight electrons. It is based on the observation that the atoms of the main group elements have a tendency to participate in chemical bonding in such a way that each atom of the resulting molecule has eight electrons in the valence shell. The octet rule is only applicable to the main group elements.
The molecules of the halogens, oxygen, nitrogen, and carbon are known to obey the octet rule. In general, the elements that obey this rule include the s-block elements and the p-block elements .
The octet rule can be observed in the bonding between the carbon and oxygen atoms in a carbon dioxide molecule, as illustrated via a Lewis dot structure below.
The shared electrons fulfil the valency requirements of both the bonded atoms. Thus, it can be noted that both the oxygen atoms and the carbon atom have an octet configuration in the CO2 molecule.
Upon observing that the noble gases were chemically inert, the electronic theory of valency was proposed by the German physicist Walther Kossel and the American chemist Gilbert Lewis. It was based on the tendency of atoms to assume the most stable state possible.
Example : Carbon Dioxide
Carbon dioxide is created through the bonding of one carbon atom and two oxygen atoms. If carbon has four electrons within its valence shell, it would need another four to become an . If oxygen had six electrons in its outer state, to become an octet it would need two.
Carbon would then share two of its valence electrons with one oxygen, and the other two valence electrons with the second oxygen. Oxygen would then share two of its electrons with the carbon.
These shared electrons then allow each of the atoms to fill their valence shells, therefore all the atoms within the molecule have reached . This is called ‘covalent‘ because the electrons are shared and not transferred.
This side of science is much more academic and less practical, however there is a much more fun and hands on approach towards science too such as our candle experiment.
If you want to read similar articles to What Is the Octet Rule in Chemistry: Explanation and Examples, we recommend you visit our Learning category.
Don’t Miss: Molecular Shape Of Ccl4
The Expanded Octet Hypervalency
Some main group elements have the ability to form hypervalent compounds. Examples include sulfur hexafluoride and phosphorus pentachloride . If all the phosphorus-chlorine bonds in a PCl5 molecule are covalent, it would imply that the phosphorus molecule is violating the octet rule by holding a total of 10 valence electrons.
The formation of five bonds by the phosphorus molecules can be explained by the sp3d hybridization in PCl5. Here, one s orbital, three p orbitals, and one d orbital undergo hybridization to form an sp3d hybrid. This hybrid orbital forms five covalent bonds with the five chlorine atoms.
The structure of the hypervalent PCl5 molecule is trigonal bipyramidal, as illustrated above.
List Four Elements That Do Not Obey The Octet Rule
Since 1p subshells do not exist, some elements find stability in 1s2 configurations. On the other hand, some elements exhibit hypervalency and have the ability to form hypervalent molecules. Some elements that disobey the octet rule include:
- Hydrogen
- Phosphorus
- Sulfur
Thus, a brief explanation of the octet rule is provided in this article along with its exceptions. To learn more about concepts related to chemical bonding, such as hybridization, register with BYJUS and download the mobile application on your smartphone.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Read Also: What Happened To Beth Thomas Biological Father