Properties And Types Of Ionic Compounds
Ionic compounds are held together by the electrostatic forces created by the attraction of the positively charged cations and negatively charged anions. These can be simple ions such as the sodium and chloride in sodium chloride, or polyatomic species such as the ammonium and carbonate ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbors, so are not considered to be part of individual molecules, but instead as part of a continuous three-dimensional network or lattice, usually in a crystalline structure. Figure 4.6 shows the structure of sodium chloride
Figure 3.8 Crystal Lattice. The crystal structure of sodium chloride, NaCl, a typical ionic compound. The purple spheres represent sodium cations, Na+, and the green spheres represent chloride anions, Cl. Halite, the mineral form of sodium chloride, forms when salty water evaportates leaving the ions behind.
Source:; Benjah-bmm27 . Lavisky, R. Both and ; Available at:
Ionic compounds containing hydrogen ions are classified as acids, and those containing hydroxide or oxide ions are classified as bases. All other ionic compounds without these ions are known as salts.; Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids, they are;most often;electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.
Monatomic Ions Vs Polyatomic Ions
In case the ion comprises of a mono or single atom, then it is known as the monatomic ion. For instance, lets consider the hydrogen iron, H+. On the other hand, if the ion comprises of two or more atoms, then it is known as the molecular or the polyatomic ion. For instance, lets consider the dichromate anion, Cr2O72-. Since, it has more than one atom, it is known as the polyatomic ion.
Introduction To The Octet Rule
Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. This is because the number of electrons is equal to the number of protons . The overall charge on the atom is zero, because the magnitude of the negative charge is the same as the magnitude of the positive charge. This one-to-one ratio of charges is not, however, the most common state for many elements. Deviations from this ratio result in charged particles calledions.
Throughout nature, things that are high in energy tend to move toward lower energy states. Lower energy configurations are more stable, so things are naturally drawn toward them. For atoms, these lower energy states are represented by the noble gas elements. These elements have electron configurations characterized by full s and p subshells. This makes them stable and unreactive. They are already at a low energy state, so they tend to stay as they are.
The elements in the other groups have subshells that are not full, so they are unstable when compared to the noble gases. This instability drives them toward the lower energy states represented by the noble gases that are nearby in the periodic table. In these lower energy states, the outermost energy level has eight electrons . The tendency of an atom toward a configuration in which it possesses eight valence electrons is referred to as the .
Figure 3.1 Periodic Table with Electron Dot Symbols.
Photograph depicted in a by:Unknown Author
Also Check: What Does Coordinate Mean In Math
Common Questions About Ionic Bonding In Chemistry
Q: What is ionic bonding?
In ionic bonding, electrons are transferred from one atom to another, resulting in the formation of positive and negative ions. Its the attraction created between positive and negative ions that creates a compound.
Q: What are some examples of ionic bonding?
There are many examples of ionic bonding in nature. Table salt is an excellent example of an ionic bond between sodium and chlorine. Silicon and oxygen also bond with a strong ionic bond to form quartz. It should be noted that the beach sand is made of quartz.
Q: What are the properties of ionic bonding?
Ionic bonding has three unique properties. These features include high resistance, electrical insulation, and transparency.
Examples Of Polyatomic Anions
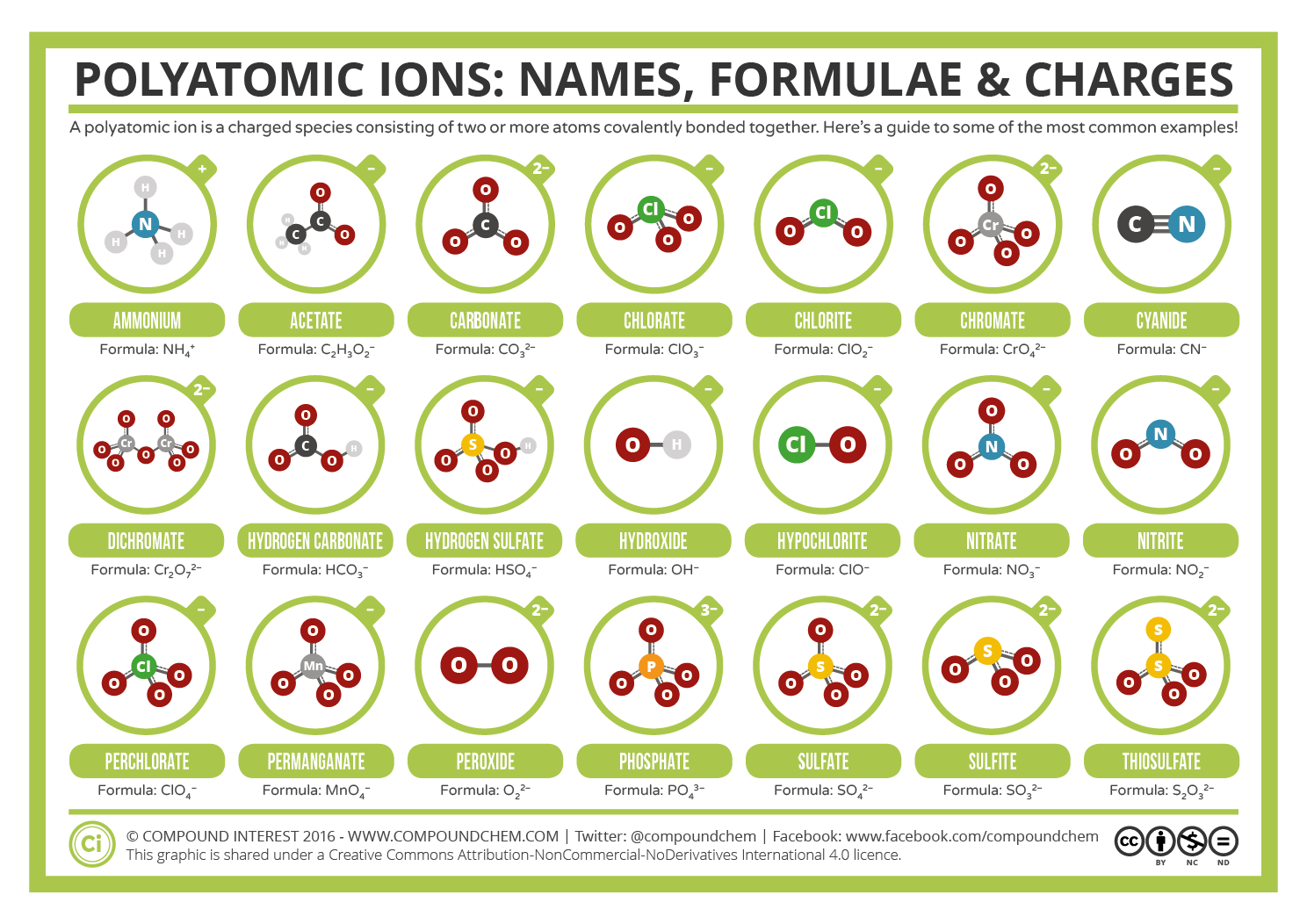
Five common examples of polyatomic anions are provided below.
- The nitrate anion, denoted by the chemical formula NO3.
- The nitrite anion, denoted by the chemical formula NO2.
- The hydroxide anion, denoted by the chemical formula OH.
- The carbonate anion, denoted by the chemical formula CO32-.
- The sulfate anion, denoted by the chemical formula SO42-.
Also Check: What Is Activation Energy Biology
Naming Ions And Ionic Compounds
Some compounds have common names, like water for H2O. However, there are thousands of other compounds that are uncommon or have multiple names. Also, the common name is usually not recognized internationally. What looks like water to you might look like agua or vatten to someone else. To allow chemists to communicate without confusion, there are naming conventions to determine the systematic name of a chemical. For the chemistry naming system in this text, we will primarily be using the International Union of Pure and Applied Chemistry naming system. Note that there is also an older and more archaic naming system, in addition to the IUPAC system. In some instances the older naming system is still in high use. These deviations from the IUPAC system will be noted throughout the text, as you will likely still see this older nomenclature still in use within chemical laboratories and the health sciences field.
For cations that have more than one charge state the name of the atom is followed by a roman numeral and then the term ion, to distinguish the different ionic states. For example, iron has two predominant ionic forms, Fe2+ and Fe3+. Thus, in naming these two ions, we would refer to the first one as the iron ion, and the second as the iron ion.; This way, there is no confusion about which ion is being referred to when discussing a compound.
Examples Of Anions Formed By Organic Compounds
Some anions are formed via the deprotonation of certain organic acids. Other anions are known to be derived from certain organic compounds. Common examples of anions that are derived from organic compounds are listed below.
- The formate anion, denoted by the chemical formula HCOO.
- The acetate anion, denoted by the chemical formula CH3COO.
- The cyanide anion, denoted by the chemical formula CN.
To learn more about the definition of ions and other related concepts such as the periodic trends in the ionization enthalpies of the elements, register with BYJUS and download the mobile application on your smartphone.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Recommended Reading: Who Can Do My Math Homework
How Does Recharging A Lithium
When the lithium-ion battery in your mobile phone is powering it, positively charged lithium ions move from the negative anode to the positive cathode. They do this by moving through the electrolyte until they reach the positive electrode. There, they are deposited. The electrons, on the other hand, move from the anode to the cathode.
When the battery is in use, the lithium ions flow from the anode to the cathode, and the electrons move from the cathode to the anode.
When you charge a lithium-ion battery, the exact opposite process happens. The lithium ions move back from the cathode to the anode. The electrons move from the anode to the cathode.
Illustration – Text Version
When the battery is charging, the lithium ions flow from the cathode to the anode, and the electrons move from the anode to the cathode.
As long as lithium ions are making the trek from one electrode to another, there is a constant flow of electrons. This provides the energy to keep your device running. Since this cycle can be repeated hundreds of times, this type of battery is rechargeable.
Did you know?
Sometimes lithium-ion batteries are referred to as “rocking chair batteries.” This is because lithium ions ‘rock’ back and forth between the electrodes.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie; no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Don’t Miss: What Is The Formula Of Volume In Physics
Ionic Bonding: Definition And Examples
Indeed, we see this type of ionic bond in many kinds of common materials, for example, sodium chloride or table salt. Whenever you have an alkali metal, like sodium, that meets up with a halogen, like fluorine or chlorine, they form a salt. The positive and negative ions attract each other, and so you get the bond. Now, because these bonds are between two ions, it is called the ionic bond.;
And there are many examples from the periodic table. Sodium chloride is the most common example of these, but there are many others. For example, when you buy salt, youll often notice that its called iodized salt. Thats because we need a small amount of iodine in our diet to help the thyroid gland.;
If we dont get it other ways, we can get it from table salt. Because in addition to having sodium chloride, table salt typically has about 1/100th of a percent of potassium iodine, another one of these alkali halides. And thats because potassium, in the periodic table, in the first column, bonds with iodine, the halogen, in the next to the last column.
Learn more about phase transformations and chemical reactions.
Waste Water Produced By Resin Regeneration
Most ion-exchange systems use columns of ion-exchange resin that are operated on a cyclic basis.
During the filtration process, water flows through the resin column until the resin is considered exhausted. That happens only when water leaving the column contains more than the maximal desired concentration of the ions being removed. Resin is then regenerated by sequentially backwashing the resin bed to remove accumulated suspended solids, flushing removed ions from the resin with a concentrated solution of replacement ions, and rinsing the flushing solution from the resin. Production of backwash, flushing, and rinsing wastewater during regeneration of ion-exchange media limits the usefulness of ion exchange for wastewater treatment.
Water softeners are usually regenerated with brine containing 10% sodium chloride. Aside from the soluble chloride salts of divalent cations removed from the softened water, softener regeneration wastewater contains the unused 50â70% of the sodium chloride regeneration flushing brine required to reverse ion-exchange resin equilibria. Deionizing resin regeneration with sulfuric acid and sodium hydroxide is approximately 20â40% efficient. Neutralized deionizer regeneration wastewater contains all of the removed ions plus 2.5â5 times their equivalent concentration as sodium sulfate.
Also Check: Is Chemistry Required To Graduate High School
What Is A Complex Ion
A complex ion has a metal ion at its center with a number of other molecules or ions surrounding it. These can be considered to be attached to the central ion by coordinate bonds The molecules or ions surrounding the central metal ion are called ligands. Simple ligands include water, ammonia and chloride ions.
What all these have got in common is active lone pairs of electrons in the outer energy level. These are used to form co-ordinate bonds with the metal ion. All ligands are lone pair donors. In other words, all ligands function as Lewis bases.
How Are Ions Created
Several methods exist for the preparation of ions. For example, spontaneous collisions between the molecules of a liquid or gaseous fluid can result in one of the electrons being knocked off an atom/molecule. This results in the formation of a positively charged ion and a free electron. This type of ionization is commonly referred to as physical ionization. The free-electron may even go on to attach itself to another atom or molecule, resulting in the formation of a new negatively charged anion.
Another important process through which ions can be created is through chemical interactions. For example, when an ionic compound such as salt is dissolved in a suitable solvent , the atoms that constitute the salt undergo dissociation and form free ions. When common salt, also known as sodium chloride, is dissolved in water, it undergoes dissociation to yield sodium cations and chloride anions. It can be noted that the sodium cations are denoted by the symbol Na+ and the chloride anions are denoted by the symbol Cl.
Other notable processes through which ions can be formed include the passage of direct currents through certain conducting solutions, which results in the formation of ions in the solution. It can also be noted that the dissolving of anodes via the process of ionization also yields large amounts of free ions.
Read Also: What Is Uneven Development In Geography
These Ions Exist In The Same Form On Both Sides Of A Chemical Reaction
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Ions are atoms or molecules that carry a net electrical charge. There are different types of ions, including cations, anions, and spectator ions. A spectator ion is one that exists in the same form on both the reactant and product sides of a chemical reaction.
What Is Difference Between Ions And Radicals Nitrogen Is Ions Or Radicals
An ion is an atom or molecule that;is positively or negatively;charged;due;to the;loss or gain of;an electron.; In order for an atom to be neutral the number of electron orbiting the nucleus must match the number of protons in the nucleus.; If these don’t match,;then we have an ion.; So anytime an atom or molecule looses or gains an electron, and the number of electrons no longer match the number of protons, then it is an;ion.; The result is this atom or molecule now carries a charge, whether it be positive or negative.
A radical is a molecule or atom that has at least one;unpaired electron , but;this moelcule or atom does not carry a charge like an ion because the number of orbiting electrons still matches the number of protons in the nucleus.; however it is very reactive.
Elemental;nitrogen has an atomic number of 7 with a mass of 14.0067.; Therefore, it has a proton number of 7 in the nucleus as determined by subtracting number of electrons from the mass number.; Because its number of electrons match the number of protons in its elemental state it cannot be an ion.
However, if we check it’s shells we get 1S2, 2S2, 2P3.; Because there is at least one;unpaired electron floating around now in the outermost shell it must be a radical.
You May Like: How To Login To Imagine Math
Acidic Basic And Neutral Solution
In pure water the concentration of hydronium ion and hydroxide ion are same and equal to 10-7;M at 250;C. This types of solution is known as neutral solution. But depending on the difference between their concentration, the solution is named as acidic or basic. Such as
- If;;= , it is a neutral solution.
- If; > , it is an acidic solution.
- If; < , it is a basic solution.
What Is An Ion Explain The Types Of Ion With Examples
Asked by Madhu
Apoorva Purohit
An ion is a positively or negatively charged atom or groups of atoms. There are two types of ions:
- An atom loses one or more electrons from its valence shell. It becomes positively charged. These positively charged atoms are called cations. For example, Sodium atomic number is 11. So, there is one electron in the valence shell. To stabilize the atom, sodium loses one electron and is a deficit of the negative charge. This will give rise to sodium ion. Na+
- When an atom gains one or more electrons into its valence shell, it becomes negatively charged. These negatively charged atoms are called anions. For example, the atomic number of Chlorine is 17. So, it has seven valence electron and requires one electron to become stable. Thus, chlorine gains one electron and becomes negatively charge. This is called a chloride ion
- ;Since there is a loss of electrons in a cation, there is a possibility f losing a shall. Hence the size of a cation is smalled.
- ;Since there is a gain of electrons for an anion, there is more number of electrons, causing the shells to repel. This si the reason why an anion is always bigger than its original atom size.;
Also Check: What Is Figure Ground Perception Psychology
Cation Vs Anion Periodic Table
It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations , whilst most other nonmetals typically form anions . However, some elements are capable of forming both cations and anions given the right conditions. One example is hydrogen, which may gain or lose an electron, forming hydride compounds such as ZnH2 and hydron compounds such as H2O .
Elements in group 18 of the periodic table the noble gases, tend not to form ions due to the arrangement of their electrons which makes them generally unreactive.
Kw Increases With Increase Of Temperature
Autoionisation of water is an endothermic process. According to Le chateliers principle, if conditions are changed in a equilibrium process, the equilibrium will shift to such a direction where it can minimize the effect of the change of the condition. Thus if water is heated the equilibrium will shift to right to form more ions by absorbing extra heat as this is an endothermic process. According to the equation of Kw, if the concentration of ions increases the;Kw increases. So we can say that Kw;increases with the increase of temperature.
Read Also: What Is Three Dimensional Geometry