Latent Heat Of Transformation
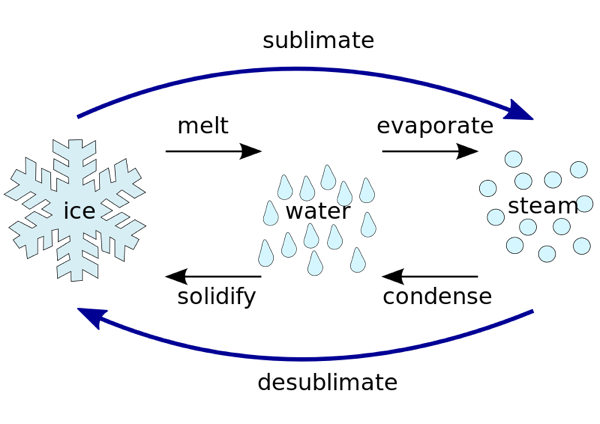
When you add heat to a solid, the temperature will rise until it reaches a line on the phase diagram. Then, instead of the temperature rising, all the heat is used in changing the phase of the substance. The heat used in that process is called the latent heat of transformation. The heat used to change a substance from a solid to a gas is called the latent heat of sublimation. Heat is absorbed in the change from solid to gas, and the same amount of heat is released in the change back from gas to solid .
From The Gnu Version Of The Collaborative International Dictionary Of English
- adjective Lifted up high in place exalted aloft uplifted lofty.
- adjective Distinguished by lofty or noble traits eminent — said of persons.
- adjective Awakening or expressing the emotion of awe, adoration, veneration, heroic resolve, etc. dignified grand solemn stately — said of an impressive object in nature, of an action, of a discourse, of a work of art, of a spectacle, etc..
- adjectivePoetic Elevated by joy elate.
- adjectivePoetic Lofty of mien haughty proud.
- noun A grand or lofty style in speaking or writing a style that expresses lofty conceptions.
- noun That which is grand in nature or art, as distinguished from the merely beautiful.
- intransitive verb To pass off in vapor, with immediate condensation specifically, to evaporate or volatilize from the solid state without apparent melting — said of those substances, like arsenic, benzoic acid, etc., which do not exhibit a liquid form on heating, except under increased pressure.
- transitive verbArchaic To raise on high.
- transitive verb To subject to the process of sublimation to heat, volatilize, and condense in crystals or powder to distill off, and condense in solid form hence, also, to purify.
- transitive verb To exalt to heighten to improve to purify.
- transitive verb To dignify to ennoble.
\ Is Always Greater Than \
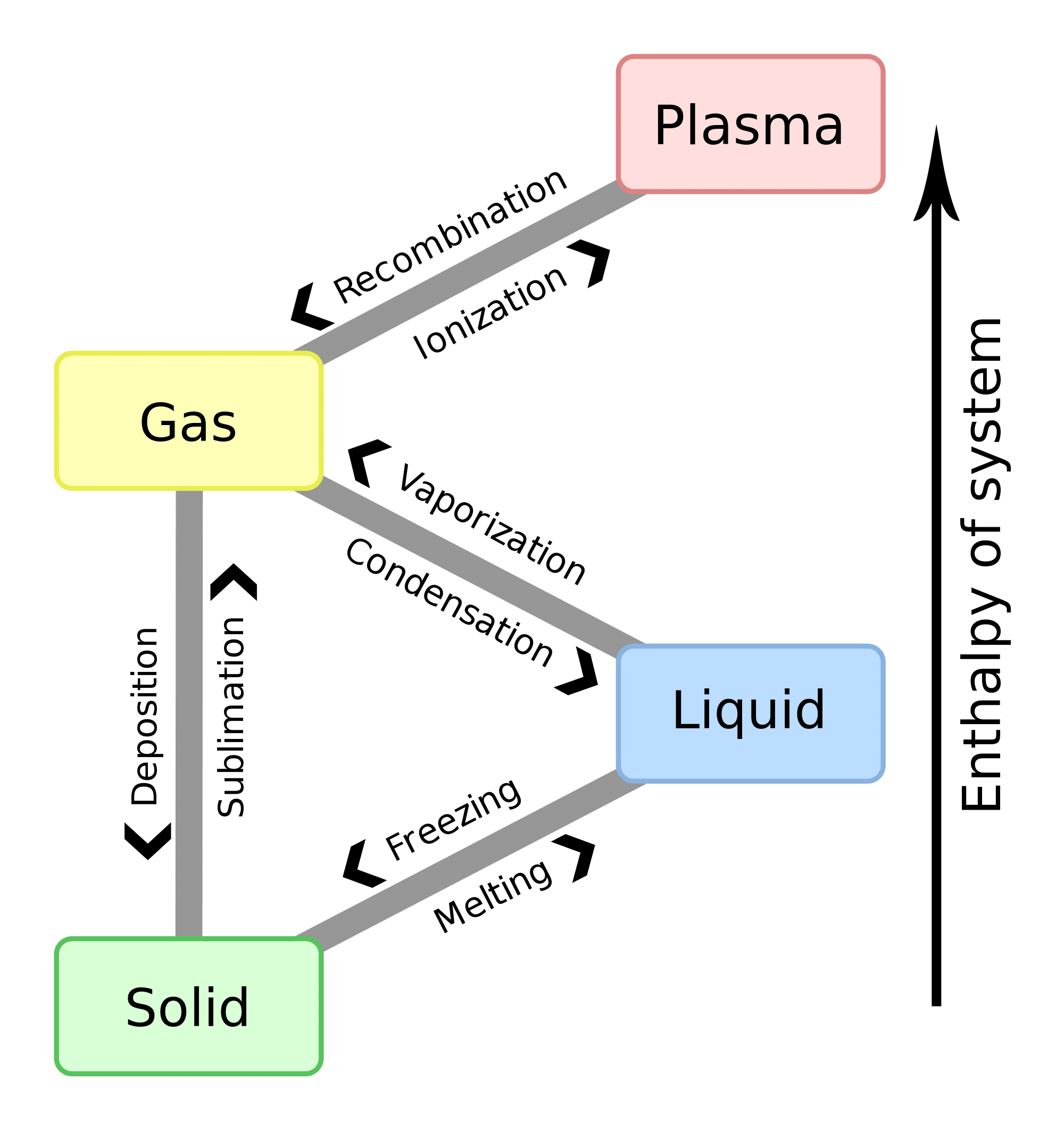
Vaporization is the transfer of molecules of a substance from the liquid phase to the gas phase. Sublimation is the transfer of molecules from the solid phase to the gas phase. The solid phase is at a lower energy than the liquid phase: that is why substances always release heat when freezing, hence \. Hence, although both sublimation and evaporation involve changing a substance into its gaseous state, the enthalpy change associated with sublimation is always greater than that of vaporization. This is because solid have less energy than those of a liquid, meaning it is takes more energy to excite a solid to its gaseous phase than it does to excite a liquid to its gaseous phase.
Another way to look this phenomena is to take a look at the different energies involved with the heat of sublimation:
Already we know that ÎEbond=ÎH*Îm and ÎEbond=ÎH*Îm. Hence, \ is actually one component of \.
Example 1
Consider the sublimation of ice:
Sublimation can be decomposed involve two steps :
- Step 1: The melting of solid water to generate liquid water
- Step 2: The evaporation of liquid water to generate gaseous water
The enthalpy change of Step 1 is the molar heat of fusion, \ and the enthalpy change of Step 2 is the molar heat of vaporization, \. Combining these two equations and canceling out anything that appears on both sides of the equation , we’re back to the sublimation equation:
Step 1 + Step 2 = Sublimation
Read Also: How To Avoid Parallax Error In Physics
Examples Of Sublimate In A Sentence
sublimatedsublimatedsublimate CNNsublimate The Conversationsublimate Forbessublimate New York Timessublimate Popular MechanicssublimateScientific Americansublimate Harper’s BAZAARsublimate Los Angeles Timessublimate Scientific AmericansublimatesScientific Americansublimates Space.comsublimates Ars Technicasublimates Space.comsublimates Ars Technica
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘sublimate.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Examples Of Sublime In A Sentence
Encyclopedia Of The Solar SystemTess of the D’UrbervillesWhy New Orleans MattersSaveurNew Republicsublimesublimesublime Timesubliming Quanta Magazinesublime Popular MechanicssublimedArs Technicasublimesandiegouniontribune.comsublime VarietysublimeThe New Yorkersublime Travel + Leisuresublime Curbedsublime EW.comsublimeForbessublime Vulturesublime Forbes
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘sublime.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Don’t Miss: Volume Formula Physics
Choose The Right Synonym For Sublime
Adjective
splendid, resplendent, gorgeous, glorious, sublime, superb mean extraordinarily or transcendently impressive. splendid implies outshining the usual or customary. the wedding was a splendid occasion resplendent suggests a glowing or blazing splendor. resplendent in her jewelrygorgeous implies a rich splendor especially in display of color. a gorgeous red dress glorious suggests radiance that heightens beauty or distinction. a glorious sunset sublime implies an exaltation or elevation almost beyond human comprehension. a vision of sublime beauty superb suggests an excellence reaching the highest conceivable degree. her singing was superb
Practical Applications Of The Heat Of Sublimation
The heat of sublimation can be useful in determining the effectiveness of medicines. Medicine is often administered in pill form, and the substances which they contain can sublime over time if the pill absorbs too much energy over time. Often times you may see the phrase “avoid excessive heat on the bottles of common painkillers . This is because in high temperature conditions, the pills can absorb heat energy, and sublimation can occur.
Read Also: Which Founding Contributors To Psychology Helped Develop Behaviorism
Sublimation Use And Examples
The most common example of sublimation is dry ice sublimating into gaseous carbon dioxide. Solid carbon dioxide will sublimate at -78.5°C at standard pressures, so a block of dry ice in the open will sublimate very noticeably, hence the use of dry ice in fog machines. The pressure of the triple point of carbon dioxide is very high, so it is hard to get gaseous carbon dioxide through the evaporation of liquid carbon dioxide.
Naphthalene is another solid substance found in moth pesticides that sublimates very easily at room temperatures as it is composed of non-polar molecules held together only by weak van der Waals forces. In general, the weaker the strength of a substances chemical bonds, the less energy input required for the solid to sublimate into a gas. Other naturally occurring materials that will sublimate at standard temperatures and pressures include iodine and arsenic. Technically though, any substance will sublimate provided it is at the right temperature and pressure.
If I melt dry ice, can I swim without getting wet? Steven Wright
ADVERTISEMENT
Why Does Camphor Sublime
At 179C camphor melts to form a liquid but the moment it melts it catches fire and starts burning. … So they sublime because their total atmospheric pressure is less than their vapor pressure, and they do not melt because the temperature at which their sublimation occurs is much less then their melting point.
Recommended Reading: Eoc Fsa Warm Ups Algebra 1 Answers
Phase Diagrams And Sublimation
The phase transitional properties of matter can be represented by a phase diagram. A phase diagram is a visual representation of how a substance changes phase. It is a 2-D graph that shows the relationship between temperature/pressure and the particular state of matter a substance will take at those temperatures and pressures. For example, at normal pressure and temperature water can exist as a solid, liquid or gas. Carbon dioxide, in contrast, can only exist as a gas at normal temperatures and pressures.
True life is lived when tiny changes occur. Leo Tolstoy
ADVERTISEMENT
The lines on the phase diagram corresponding to points where a substance will undergo a phase transition from one state to another. Therefore, in the above picture, sublimation refers to any transition between the two sections divided by the bottom line. At normal pressures and temperatures, most compounds would have to transition through a liquid state to go from a solid to a gas. However, due to effects from the vapor pressure of a substance, many common substances like water will sublimate at appreciably low temperatures at normal atmospheric pressures.
Definition Of Deposition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
The settling of particles or sediment onto a surface. The particles may originate from a vapor, solution, a suspension, or a mixture.
Deposition also refers to the phase change from gas to solid.
Recommended Reading: Why Are There Different Branches Of Chemistry
Difference Between Sublime And Sublimate
September 20, 2018 Posted by Madhu
The key difference between sublime and sublimate is that the sublime refers to the verb describing the sublimation process whereas the sublimate refers to a noun that describes the end product of sublimation.
Sublimation is the process of the transition from a solid phase into a gas phase directly, without going through a liquid phase. This is an endothermic phase transition reaction in chemistry. When considering the sublimation process, it takes place at temperatures and pressures below the triple point of a substance. The triple point of a substance is the temperature and pressure at which that substance can coexist in all three phases of matter in thermodynamic equilibrium. Desublimation, on the other hand, refers to the conversion of gaseous phase back to the solid phase directly without going through a liquid phase. The two terms sublime and sublimate describe two different points of the sublimation process as stated above, in the key difference.
Summary Sublime Vs Sublimate
Difference between the two terms sublime and sublimation lies upon the use of these terms. Therefore, we can give the difference between sublime and sublimate as that the term sublime refers to the verb describing the sublimation process whereas the term sublimate refers to a noun that describes the end product of sublimation.
Reference:
1. Helmenstine, Anne Marie, Ph.D. Sublimation Definition . ThoughtCo, Jun. 22, 2018. Available here
Image Courtesy:
1.Nickelocen an einem KühlfingerBy MSchiffrer Own work, via Commons Wikimedia 2.Dry Ice Vapor By Tony Webster from Mountain View, California Dry Ice Vapor, via Commons Wikimedia
You May Like: Evaluating Functions Worksheet Algebra 2 Answer Key
From The American Heritage Dictionary Of The English Language 5th Edition
- adjective Characterized by nobility majestic.
- adjective Of high spiritual, moral, or intellectual worth.
- adjective Not to be excelled supreme.
- adjective Inspiring awe impressive.
- adjectiveArchaic Raised aloft set high.
- adjectiveArchaic Of lofty appearance or bearing haughty.
- noun Something sublime.
- intransitive verb To render sublime.
- intransitive verbChemistry To cause to sublimate.
- intransitive verb To sublimate.
What Does Sublimation Mean In Science
Sometimes, it’s easy to discern the meaning of words in science because they share some aspect of their meanings with everyday English. Scientific concepts like energy, force and even natural selection are mostly extensions of our common understanding and their colloquial meanings. Not so for sublimation. Even if you know the non-scientific meaning of the word, that knowledge won’t help you when it comes to its meaning in science. In science, sublimation has to do with the branch of physics and chemistry called thermodynamics.
Also Check: The Segment Addition Postulate Answer Key With Work
Practical Applications Of Sublimation
- Sublimation and erosion cause ablation, a process that wears down glaciers.
- Sublimation of iodine may be used to reveal latent fingerprints on paper.
- Sublimation is used to purify compounds. It is especially useful for organic compounds.
- Because dry ice sublimates so readily, the compound is used to produce fog effects.
What Is The Difference Between Sublime And Sublimate
The term sublime is a verb that we use to name the process of sublimation whereas the term sublimate refers to the chemical product that we obtain from the process of sublimation. When considering the use of these two terms, the sublime is a verb while sublimate is a noun. This is because the term sublime refers to the sublimation process whereas the term sublimate refers to the gaseous products that form at the end of the sublimation process.
The below infographic presents the difference between sublime and sublimate in tabular form for quick reference.
Also Check: What Does Relationship Mean In Math Terms
Where Does The Added Energy Go
Energy can be observed in many different ways. As shown above, ÎEtotcan be expressed as ÎEthermal +ÎEbond. Another way in which ÎEtotcan be expressed is change in potential energy, ÎPE, plus change in kinetic energy, ÎKE. Potential energy is the energy associated with random movement, whereas kinetic energy is the energy associated with velocity . ÎEtot = ÎEthermal +ÎEbond and ÎEtot = ÎPE + ÎKE are related by the equations
ÎPE = ÎEthermal +ÎEbondÎKE = ÎEthermal
for substances in the solid and liquid states. Note that ÎEthermal is divided between ÎPE and ÎKE for substances in the solid and liquid states. This is because the intermolecular and intramolecular forces that exist between the atoms of the substance have not yet been dissociated and prevent the atomic particles from moving freely about the atmosphere . Potential energy is just a way to have energy, and it generally describes the random movement that occurs when atoms are forced to be close to one another. Likewise, kinetic energy is just another way to have energy, which describes an atom’s vigorous struggle to move and to break away from the group of atoms. The thermal energy that is added to the substance is thus divided equally between the potential and the kinetic energies because all aspects of the atoms’ movement must be excited equally
What Is Sublimation In Chemistry
Sublimation in chemistry refers to the phase transition in which matter changes state from a solid immediately into a gas, without passing through an intermediate liquid phase. Sublimation occurs when atmospheric pressure is too low for a substance to exist in liquid form. Sublimation is the inverse of deposition, the phase transition in which gas goes immediately to a solid state. Whether or not a substance will sublimate from a solid to a gaseous state depends on the triple point of the substance, the temperature and pressure at which a substance exists in equilibrium as the three states of matter.
A substance will only sublimate at temperatures and pressures lower than the substances triple point.
Life is water, dancing to the tune of solids. Albert Szent-Gyorgyi
Read Also: What Is Secondary Succession In Biology
What Is The Definition Of Sublime In Science
4.3/5Chemistry
Simply so, what is an example of sublime?
The definition of sublime is something majestic, impressive or intellectually valuable. An example of sublime is a beautifully presented, formal six course meal.
Furthermore, what is a sublime look? In common use, sublime is an adjective meaning “awe-inspiringly grand, excellent, or impressive,” like the best chocolate fudge sundae you’ve ever had.
Just so, how do you use the word sublime?
sublime Sentence Examples
What is the synonym of sublime?
Synonyms: empyreal, rarified, noble-minded, empyrean, elevated, lofty, idealistic, reverend, exalted, high-minded, high-flown, grand, rarefied. Antonyms: inglorious, ignoble, profane, dejected, secular. reverend, sublime
What Is Sublimation

The term sublimation is the passage or the transformation or conversion that substances undergo when passing from one state to another, for example from a solid substance to gas.
We can define sublimation as the transition of a substance from the solid phase to the gaseous phase without changing into the liquid phase. This process is an endothermic phase transition that occurs at a temperature and pressure below the triple point of the substance. Desublimation or deposition is the reverse of this process in which a gas is directly converted into solid-state.
- Elements and compounds mainly possess three different states at various temperatures.
- The transition from solid state to gaseous state requires a transition of solid-state to liquid state and liquid state to a gaseous state.
- If solids possess sufficient vapour pressure at a particular temperature than they can directly sublime into the air.
- Solids which have high pressure at their triple point show sublimation.
- The triple point is the point at which the pressure and temperature of the substance are such that it can exist in all three states of matter simultaneously. The triple point is a characteristic point of a substance.
- There are various examples of sublimation which are experienced by us in our everyday life.
You May Like: What Are Dyes
What Is Sublimate
The term sublimate refers to the chemical product that we obtain from the process of sublimation. This means, sublimate is the name we give to the end product of the sublimation process.
Figure 02: Sublimate of Dry Ice is Carbon Dioxide Vapour
Usually, the sublimate is a gaseous compound because always the sublimation forms a gaseous product out of the solid product. For example, the sublimate of the sublimation of the dry ice is carbon dioxide vapour.
What Is Sublime
The term sublime is a verb that we use to name the process of sublimation. When we say we are going to sublime a substance, that means we are going to convert the phase of that substance from its solid phase to the gas phase without passing through its liquid phase.
Figure 01: Sublimed and Freshly Deposited Nickelocene
For example, dry ice undergoes sublimation at room temperature and pressure to form carbon dioxide vapour. There we say, dry ice sublimes to produce carbon dioxide vapour.
Also Check: Find The Length Indicated Geometry
Did You Know
Verb
To sublimate is to change the form, but not the essence. Physically speaking, it means to transform solid to vapor psychologically, it means changing the outlet, or means, of expression from something base and inappropriate to something more positive or acceptable. The word sublimate comes from the Latin verb sublimare, which means “to lift up” or “raise” and which is also the ancestor of our sublime. “Sublimate” itself once meant “to elevate to a place of dignity or honor” or “to give a more elevated character to,” but these meanings are now obsolete.