Fatty Acids And Triacylglycerides
The fatty acids are lipids that contain long-chain hydrocarbons terminated with a carboxylic acid functional group. Because the long hydrocarbon chain, fatty acids are hydrophobic or nonpolar. Fatty acids with hydrocarbon chains that contain only single bonds are called saturated fatty acids because they have the greatest number of hydrogen atoms possible and are, therefore, saturated with hydrogen. Fatty acids with hydrocarbon chains containing at least one double bond are called unsaturated fatty acids because they have fewer hydrogen atoms. Saturated fatty acids have a straight, flexible carbon backbone, whereas unsaturated fatty acids have kinks in their carbon skeleton because each double bond causes a rigid bend of the carbon skeleton. These differences in saturated versus unsaturated fatty acid structure result in different properties for the corresponding lipids in which the fatty acids are incorporated. For example, lipids containing saturated fatty acids are solids at room temperature, whereas lipids containing unsaturated fatty acids are liquids.
A triacylglycerol, or triglyceride, is formed when three fatty acids are chemically linked to a glycerol molecule . Triglycerides are the primary components of adipose tissue , and are major constituents of sebum . They play an important metabolic role, serving as efficient energy-storage molecules that can provide more than double the caloric content of both carbohydrates and proteins.
NOTE
Chemical Bonds In Biological Molecules
There are three important chemical bonds in biological molecules: covalent bonds, hydrogen bonds, and ionic bonds.
Before explaining each of them, it is important to recall the structure of the atoms that are the building blocks of molecules.
Fig. 2 – Atomic structure of carbon
Figure 2 shows the atomic structure of carbon. You can see the nucleus . Neutrons have no electrical charge, while protons have a positive charge. Therefore, overall a nucleus will have a positive charge.
Electrons orbit the nucleus and have a negative charge.
Why is this important? It is helpful to know that electrons are negatively charged, and they orbit the nucleus, in order to understand how different molecules are bound on an atomic level.
Why Are Macromolecules Important To The Human Body
Such as metabolism to supply the energy and materials needed to sustain life, transfer genetic information, control embryo differentiation, promote growth and development, and generate immune functions. Human research on biological macromolecules has gone through a long history of nearly two centuries.
Also Check: Geometry Builder 17 Answer Key
Classification Of Biological Molecules
Structurally a biomolecule is categorized by its function. They can be categorized as carbohydrates, nucleic acids, lipids, and proteins. Proteins are found in our body’s cells and account for 20% of the biomass on earth. Carbohydrates are a major source of energy for humans and act as a major source of food energy. Nucleic acids like DNA and RNA can make up chromosomes or other parts of the genome found in living things.
Types Of Biological Macromolecules
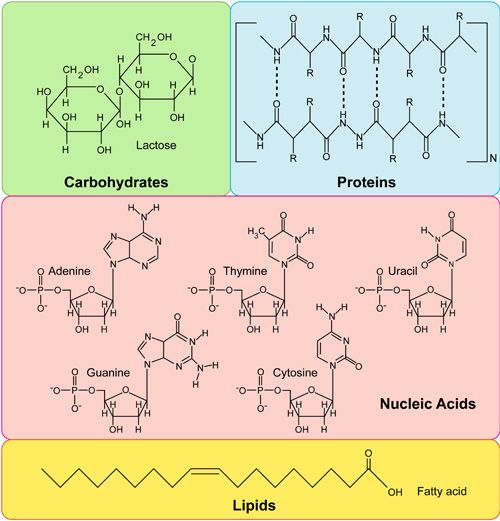
- Identify the four major classes of biological macromolecules
Key Points
- Biological macromolecules are important cellular components and perform a wide array of functions necessary for the survival and growth of living organisms.
- The four major classes of biological macromolecules are carbohydrates, lipids, proteins, and nucleic acids.
Terms
- polymerA relatively large molecule consisting of a chain or network of many identical or similar monomers chemically bonded to each other.
- monomerA relatively small molecule that can form covalent bonds with other molecules of this type to form a polymer.
Living organisms are made up of chemical building blocks
Recommended Reading: What Are Careers For Psychology Majors
Comparing The Biological Macromolecules
| Storage Signals Structural Contractile Defensive Enzyme Transport Receptors | |||
| Lipids |
Greater than 2:1 H:O |
Fatty acid and glycerol | Energy storage Protection Chemical messengers Repel water |
| Carbohydrates | Glucose, Fructose, Starch, Glycogen, Cellulose | Energy storage Structure | |
| Genetic information |
What Are The Different Types Of Biomolecules In The Body
Role and Types of Biomolecules in the Human Body. Living systems are made up of various complex biomolecules like carbohydrates, proteins, nucleic acids, lipids, etc. In addition to that some simple molecules like vitamins and mineral salts also play an important role in the functions of organisms.
Don’t Miss: Nys Common Core Algebra 1
Four Classes Of Biological Molecules
Biological molecules include the following :
- Carbohydrates are sugar molecules that are composed of hydrogen, oxygen, and carbon atoms. These are substances that produce aldehydes or ketones when they are hydrolyzed.
The number of constituent sugar units recovered following hydrolysis is used to classify monosaccharides, such as monosaccharide contain one unit, oligosaccharides contains two to ten units, and polysaccharides contain more than ten units. Thus, polysaccharides are sugars that are made up of a lengthy chain of monosaccharides.
- Polypeptide chains, which are made up of amino acids, make up proteins. The amino and carboxylic groups of 20 distinct amino acids are linked by a peptide bond. In some circumstances, the structure of a protein can be classed as primary, secondary, tertiary, or quaternary.
- Lipids are connected to fatty acids and are used by living organisms for energy and other chemical processes. Fat, oils, and hormones are some of the examples of lipids. These chemical compounds are soluble in organic solvents but not in water.
- Polynucleotides are the building blocks of nucleic acids. The three chemically different components of nucleic acid are heterocyclic base, polysaccharides , and phosphate or phosphoric acid.
Does A Technical Umbrella Term Exist That Covers The Different Kinds Of Biomolecules
I want to write a sentence along the lines of:
There are four ____ in biochemistry: proteins, lipids, carbohydrates and nucleic acids.
Does a specific technical term exist that I can use to fill the void? Or am I relatively free in my choice?
- $\begingroup$Refer to them like what? Group or type of chemical compounds? Why not? You’re asking about basic semantics…$\endgroup$ MithoronNov 27, 2016 at 19:37
- $\begingroup$Yes, I want to write a sum about the topic but I don’t know the terminology. Refer to them like groups. I mean to ask if I can divide the main biochemical molecules to 4 families.$\endgroup$Nov 27, 2016 at 20:14
- 1$\begingroup$Well, they are already divided – these are 4 groups of compounds. Group or type is better word then family here IMO, but what’s here to ask about…$\endgroup$Nov 27, 2016 at 20:20
- $\begingroup$Are they the only groups of compound in biochemistry?$\endgroup$Nov 27, 2016 at 20:33
I dont know what your background is. Maybe it is organic chemistry, where the term functional group is used in a very specific way and using what would seem to be a synonym instead is wrong. Maybe it is biology where it makes a big difference if a group is an order, a family, a genus or something else.
The typical primary metabolites in biochemistry carbohydrates, peptides/proteins, nucleic acids and lipids do not have an overarching, widely accepted technical umbrella term. You can call them groups, classes, kinds or even types and be well understood.
Read Also: Geometry Solve For X Calculator
The Biological Building Blocks
The cell is the basic unit of life. All organisms are composed of one or more cells. As will be discussed later, humans are made up of many millions of cells. In order to understand what goes wrong in cancer, it is important to understand how normal cells work. The first step is to discuss the structure and basic functions of cells.
First we will introduce the common building blocks of cells. All cells, regardless of their function or location in the body, share common features and processes. Amazingly, cells are comprised almost entirely of just four basic types of molecules. Shown above is a cell surrounded by examples of these building block molecules.
Since they are present in living things these building blocks are called biomolecules. The next sections describe the structures and functions of each of these basic building blocks. Further information on the topics on this page can also be found in most introductory Biology textbooks, we recommend Campbell Biology, 11th edition.1
Structure And Functions Of 3 Types Of Carbohydrates
Carbohydrates are the most abundant type of biological molecules.
Monomers of carbohydrates, monosaccharides, are simple sugars, and their primary role is to provide energy. For example, the brain requires a constant supply of sugar to meet its energy needs.
Structural elements of the cell walls in plants and bacteria and cell to cell communications depend on complex carbohydrate polymers such as oligosaccharides and polysaccharides.
You May Like: Different Kinds Of Lines In Geometry
What Are The Four Main Types Or Classes Of Organic Compounds Found In All Living Things Differentiate Each Type
There are four main types, or classes, of organic compounds found in all living things: carbohydrates, lipids, proteins, and nucleic acids. In addition, there are other organic compounds that may be found in or produced by some organisms.
What are the four main classes of biomolecules?
Biomolecules have a wide range of sizes and structures and perform a vast array of functions. The four major types of biomolecules are carbohydrates, lipids, nucleic acids, and proteins.
Where are the 4 macromolecules found?
The four types of macromolecules in biology are: lipids, carbohydrates, proteins and nucleic acids. There are two types of nucleic acids: DNA and RNA. In the case of eukaryotic cells, during the majority of the cell cycle, DNA is located in the nucleus. RNA is located in the nucleus and the cytoplasm.
Biological Function Of Carbohydrates
Plants and algae produce millions of tons of carbohydrates each year through photosynthesis.
The main function of carbohydrates is to provide energy, particularly through glucose.
During cellular respiration, glucose is broken down and oxidized within cells. This process is used to synthesize adenosine triphosphate the source of energy for cellular reactions.
When the quantity of adenosine triphosphate are sufficient, simple carbohydrates are converted to carbohydrate polymers or fat and stored.
Carbohydrates also have other important functions in all living organisms.
For example, they serve as building materials within the plant cells and perform cell-to-cell identification when attached to the external surfaces of the cytoplasmic membrane.
Don’t Miss: What Is Iupac In Chemistry
What Is Monounsaturated
Don’t forget about the age old question of What are the three classes of matter?
Which of the four are made and broken down by dehydration synthesis and hydrolysis, respectively? What is removed/added? What features must the building blocks have?
Know the three subgroups of lipids and their function.
fats phospholipids steroids
Know the building blocks of a fat.
Triglyceride= glycerol + 3 fatty acid tails tails are non polar
Know the building blocks of a phospholipid.
= phosphate + glycerol + 2 fatty acid tails
Relate the structure of phospholipids to their function in biological membranes.
Phospholipid bilayers = basis for biological membranes provides barrier around cells and sub cellular spaces its hydrophobic core impenetrable to most molecules solubility in H2O depends on whether substance has enough polar bonds with tightly held electrons distributed across its structure
Dna And Rna Structure
DNA structure is dominated by the well-known double helix formed by Watson-Crick base-pairing of C with G and A with T. This is known as B-form DNA, and is overwhelmingly the most favorable and common state of DNA its highly specific and stable base-pairing is the basis of reliable genetic information storage. DNA can sometimes occur as single strands or as A-form or Z-form helices, and occasionally in more complex 3D structures such as the crossover at Holliday junctions during DNA replication.
RNA, in contrast, forms large and complex 3D tertiary structures reminiscent of proteins, as well as the loose single strands with locally folded regions that constitute messenger RNA molecules. Those RNA structures contain many stretches of A-form double helix, connected into definite 3D arrangements by single-stranded loops, bulges, and junctions. Examples are tRNA, ribosomes, ribozymes, and riboswitches. These complex structures are facilitated by the fact that RNA backbone has less local flexibility than DNA but a large set of distinct conformations, apparently because of both positive and negative interactions of the extra OH on the ribose. Structured RNA molecules can do highly specific binding of other molecules and can themselves be recognized specifically in addition, they can perform enzymatic catalysis .
You May Like: What Does Lithosphere Mean In Geography
Biologically Important Properties Of Water
Water is a polar molecule. This means that one side of the molecule has a slight positive charge while the other side has a slight negative charge. These opposite charges create a force of attraction between water molecules. Furthermore, water dissolves many substances because many different types of bonds can form between the molecules in water and other substances.
Anomeric Forms Of Glucose
Fischers brilliant elucidation of the configuration of glucose did not remove all uncertainty concerning its structure. Two different crystalline forms of glucose were reported in 1895. Each of these gave all the characteristic reactions of glucose, and when dissolved in water equilibrated to the same mixture. This equilibration takes place over a period of many minutes, and the change in optical activity that occurs is called mutarotation. These facts are summarized in the diagram below.
When glucose was converted to its pentamethyl ether , two different isomers were isolated, and neither exhibited the expected aldehyde reactions. Acid-catalyzed hydrolysis of the pentamethyl ether derivatives, however, gave a tetramethyl derivative that was oxidized by Tollens reagent and reduced by sodium borohydride, as expected for an aldehyde. These reactions will be displayed above by clicking on the diagram.
Second, a pentamethyl ether derivative of the pyranose structure converts the hemiacetal function to an acetal. Acetals are stable to base, so this product should not react with Tollens reagent or be reduced by sodium borohydride. Acid hydrolysis of acetals regenerates the carbonyl and alcohol components, and in the case of the glucose derivative this will be a tetramethyl ether of the pyranose hemiacetal. This compound will, of course, undergo typical aldehyde reactions. By clicking on the diagram a second time this relationship will be displayed above.
You May Like: Where Can I Study Geography
Why Are Vitamins Called Macronutrients
Macronutrients include protein, fats, and carbohydrates that provide bulk energy, while micronutrients include vitamins and minerals required for growth and disease prevention .
What is the most essential biomolecule for human body?
Lipids are the responsible for energy storage in a cell and are the major component of the cell membrane. Among all these biomolecules, I would pick nucleic acids as the most important for life. There are two types of nucleic acids: DNA and RNA .
What are the four major classes of biological molecules?
There are four major classes of biological macromolecules , and each is an important component of the cell and performs a wide array of functions. Combined, these molecules make up the majority of a cells mass.
What kind of molecules are necessary for life?
The large molecules necessary for life that are built from smaller organic molecules are called biological macromolecules. There are four major classes of biological macromolecules , and each is an important component of the cell and performs a wide array of functions.
What Are The 4 Major Biological Molecules And Why Are They Important
There are four major classes of biological macromolecules each is an important cell component and performs a wide array of functions. Combined, these molecules make up the majority of a cells dry mass .
What is the structure and function of biomolecules?
Biomolecule, also called biological molecule, any of numerous substances that are produced by cells and living organisms. Biomolecules have a wide range of sizes and structures and perform a vast array of functions. The four major types of biomolecules are carbohydrates, lipids, nucleic acids, and proteins.
Recommended Reading: What Does Range In Math
Importance Of Biological Molecules
Molecules are all around us and they play a crucial role in our everyday lives. They are the building blocks of cells and can be very simple or very complex. One such molecule is DNA which is comprised of nucleotides, sugars, phosphates and nitrogenous bases: Adenine , Cytosine , Guanine and Thymine . These bases come together in a specific sequence along the molecule’s length giving the coding for an organism’s unique genetic information.
Four Major Types Of Biomolecules
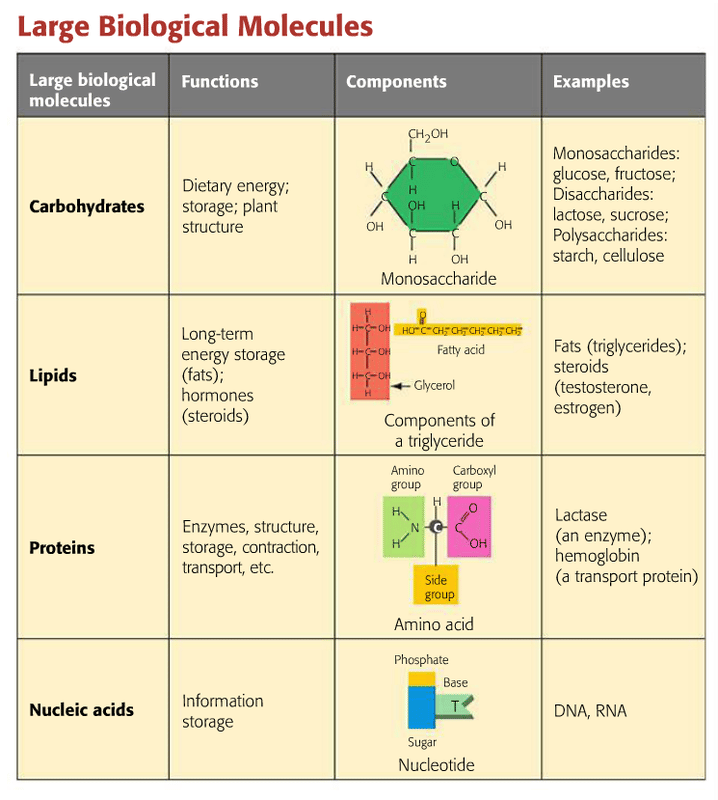
Approximately 10,000 to 100,000 molecules are present in a cell to regulate bodily function. But the four major types of biomolecules include carbohydrates, lipids, nucleic acids, and proteins. Most of the other compounds are derivatives of these major primary compounds.
Every biomolecule has its characteristics and is designated to perform some specific function essential for life. So, lets see what they are all about!!!
1. Carbohydrates
Carbohydrates are a vital part of a healthy diet. They provide the energy required to do work. Scientifically, its a polyhydroxy aldehyde or polyhydroxy ketone. Carbohydrates are the most abundant biomolecules on earth.
Types of Carbohydrates and Their Functions
Depending on the number of products formed after hydrolysis, carbohydrates are classified into three groups.
| No. | |
|---|---|
| Glucose and Glucose | A constituent of plant glycosides and some polysaccharides |
You May Like: How To Solve Normality Problems In Chemistry
Pmc Mdcat Mcqs On Biological Molecules
PMC MDCAT MCQs On Biological Molecule
Biomolecules are biologically important organic compounds, or, more generally, one of the major classes of chemical compounds found in living organisms. The five major classes of biomolecules are carbohydrates, lipids, proteins, nucleic acids and polysaccharides some definitions also include ions and organic solvents. Living organisms are made from these same biomolecules, but other non-living substances of similar structure can be made artificially as well. In humans, most biomolecules are made up of carbon, hydrogen, and oxygen atoms.
$title=
Biological Polymers: Proteins Carbohydrates Lipids
- B.A., Biology, Emory University
- A.S., Nursing, Chattahoochee Technical College
Biological polymers are large molecules composed of many similar smaller molecules linked together in a chain-like fashion. The individual smaller molecules are called monomers. When small organic molecules are joined together, they can form giant molecules or polymers. These giant molecules are also called macromolecules. Natural polymers are used to build tissue and other components in living organisms.
Generally speaking, all macromolecules are produced from a small set of about 50 monomers. Different macromolecules vary because of the arrangement of these monomers. By varying the sequence, an incredibly large variety of macromolecules can be produced. While polymers are responsible for the molecular “uniqueness” of an organism, the common monomers are nearly universal.
The variation in the form of macromolecules is largely responsible for molecular diversity. Much of the variation that occurs both within an organism and among organisms can ultimately be traced to differences in macromolecules. Macromolecules can vary from cell to cell in the same organism, as well as from one species to the next.
Recommended Reading: What Not To Do In The Chemistry Lab